Abstract
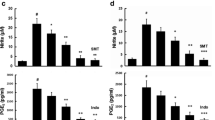
Accumulating evidence suggests that inflammatory processes in the central nervous system that are mediated by microglial activation play important roles in several neurodegenerative disorders. Therefore, development of methods for microglial inhibition is considered an important strategy in the search for neuroprotective agents. Caffeic acid phenethyl ester (CAPE) is distributed wildly in nature, but rapid decomposition by esterase leads to its low bioavailability. In this study, we investigated the effects of KS370G, a novel caffeic acid phenylethyl amide, on microglial activation. KS370G significantly inhibited the release of nitric oxide (NO) and the expressions of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Treatment with KS370G also induced heme oxygenase (HO)-1 and suppressors of cytokine signaling (SOCS)-3 expression in the microglia. Furthermore, the anti-inflammatory effects of KS370G were found to be regulated by phosphorylated adenosine monophosphate-activated protein kinase-α (AMPK-α) translocated to the nucleus. Moreover, KS370G showed significant anti-neuroinflammatory effects on microglial activation in vivo and on motor behavior as well. The protective effect of KS370G was weakened by an AMPK inhibitor Compound C. This study focuses on the importance of key molecular determinants of inflammatory homeostasis, AMPK, HO-1, and SOCS-3, and their possible involvement in anti-neuroinflammatory responses.






Similar content being viewed by others
References
Olson JK, Miller SD (2004) Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol 173(6):3916–3924
Weiner HL (2009) The challenge of multiple sclerosis: how do we cure a chronic heterogeneous disease? Ann Neurol 65(3):239–248. doi:10.1002/ana.21640
Politis M, Pavese N, Tai YF, Kiferle L, Mason SL, Brooks DJ, Tabrizi SJ, Barker RA, Piccini P (2011) Microglial activation in regions related to cognitive function predicts disease onset in Huntington's disease: a multimodal imaging study. Hum Brain Mapp 32(2):258–270. doi:10.1002/hbm.21008
Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R (2007) Inflammation in Parkinson's diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des 13(18):1925–1928
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22(20):7004–7014
Loihl AK, Murphy S (1998) Expression of nitric oxide synthase-2 in glia associated with CNS pathology. Prog Brain Res 118:253–267
Bal-Price A, Brown GC (2001) Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci 21(17):6480–6491
Beckman JS, Chen J, Crow JP, Ye YZ (1994) Reactions of nitric oxide, superoxide and peroxynitrite with superoxide dismutase in neurodegeneration. Prog Brain Res 103:371–380
Chao CC, Hu S, Peterson PK (1995) Modulation of human microglial cell superoxide production by cytokines. J Leukoc Biol 58(1):65–70
Wang JY, Shum AY, Ho YJ, Wang JY (2003) Oxidative neurotoxicity in rat cerebral cortex neurons: synergistic effects of H2O2 and NO on apoptosis involving activation of p38 mitogen-activated protein kinase and caspase-3. J Neurosci Res 72(4):508–519. doi:10.1002/jnr.10597
Liang X, Wu L, Wang Q, Hand T, Bilak M, McCullough L, Andreasson K (2007) Function of COX-2 and prostaglandins in neurological disease. J Mol Neurosci 33(1):94–99
Le WD, Xie WJ, Appel SH (1999) Protective role of heme oxygenase-1 in oxidative stress-induced neuronal injury. J Neurosci Res 56(6):652–658. doi:10.1002/(SICI)1097-4547(19990615)56:6<652::AID-JNR11>3.0.CO;2–5
Platt JL, Nath KA (1998) Heme oxygenase: protective gene or Trojan horse. Nat Med 4(12):1364–1365. doi:10.1038/3947
Otterbein LE, Choi AM (2000) Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol 279(6):L1029–L1037
Lu DY, Leung YM, Su KP (2012) Interferon-alpha induces nitric oxide synthase expression and haem oxygenase-1 down-regulation in microglia: implications of cellular mechanism of IFN-alpha-induced depression. Int J Neuropsychopharmacol/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum: 1–12. doi:10.1017/S1461145712000338
Lu DY, Tsao YY, Leung YM, Su KP (2010) Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 35(11):2238–2248. doi:10.1038/npp.2010.98
Lu DY, Chen JH, Tan TW, Huang CY, Yeh WL, Hsu HC (2013) Resistin protects against 6-hydroxydopamine-induced cell death in dopaminergic-like MES23.5 cells. J Cell Physiol 228 (3):563--571. doi:10.1002/jcp.24163
Lin HY, Yeh WL, Huang BR, Lin C, Lai CH, Lin H, Lu DY (2012) Desipramine protects neuronal cell death and induces heme oxygenase-1 expression in Mes23.5 dopaminergic neurons. PloS one 7 (11):e50138. doi:10.1371/journal.pone.0050138
Choi AM, Alam J (1996) Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol 15(1):9–19
Lee TS, Chau LY (2002) Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8(3):240–246. doi:10.1038/nm0302-240
Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S (2001) Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci U S A 98(15):8798–8803. doi:10.1073/pnas.161272598
Terry CM, Clikeman JA, Hoidal JR, Callahan KS (1998) Effect of tumor necrosis factor-alpha and interleukin-1 alpha on heme oxygenase-1 expression in human endothelial cells. Am J Physiol 274(3 Pt 2):H883–H891
Yoshimura A, Naka T, Kubo M (2007) SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol 7(6):454–465. doi:10.1038/nri2093
Wong PK, Egan PJ, Croker BA, O'Donnell K, Sims NA, Drake S, Kiu H, McManus EJ, Alexander WS, Roberts AW, Wicks IP (2006) SOCS-3 negatively regulates innate and adaptive immune mechanisms in acute IL-1-dependent inflammatory arthritis. J Clin Invest 116(6):1571–1581. doi:10.1172/JCI25660
Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA (2004) AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem 279(46):47898–47905. doi:10.1074/jbc.M408149200
Leclerc I, Rutter GA (2004) AMP-activated protein kinase: a new beta-cell glucose sensor?: regulation by amino acids and calcium ions. Diabetes 53(3):S67–S74
Hardie DG, Carling D, Carlson M (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem 67:821–855. doi:10.1146/annurev.biochem.67.1.821
Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D (2005) Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2(1):21–33. doi:10.1016/j.cmet.2005.06.005
Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2(1):9–19. doi:10.1016/j.cmet.2005.05.009
Hurley RL, Barre LK, Wood SD, Anderson KA, Kemp BE, Means AR, Witters LA (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J Biol Chem 281(48):36662–36672. doi:10.1074/jbc.M606676200
Raney MA, Turcotte LP (2008) Evidence for the involvement of CaMKII and AMPK in Ca2+-dependent signaling pathways regulating FA uptake and oxidation in contracting rodent muscle. J Appl Physiol 104(5):1366–1373. doi:10.1152/japplphysiol.01282.2007
Saitoh M, Nagai K, Nakagawa K, Yamamura T, Yamamoto S, Nishizaki T (2004) Adenosine induces apoptosis in the human gastric cancer cells via an intrinsic pathway relevant to activation of AMP-activated protein kinase. Biochem Pharmacol 67(10):2005–2011. doi:10.1016/j.bcp.2004.01.020
Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I (2004) 5-Aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci 24(2):479–487. doi:10.1523/JNEUROSCI.4288-03.2004
Lu DY, Tang CH, Chen YH, Wei IH (2010) Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem 110(3):697–705. doi:10.1002/jcb.22580
Gulcin I (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86(3):345–391. doi:10.1007/s00204-011-0774-2
Hwang JT, Kwon DY, Yoon SH (2009) AMP-activated protein kinase: a potential target for the diseases prevention by natural occurring polyphenols. New Biotechnology 26(1–2):17–22. doi:10.1016/j.nbt.2009.03.005
Visioli F, Davalos A (2011) Polyphenols and cardiovascular disease: a critical summary of the evidence. Mini Rev Med Chem 11(14):1186–1190
Guo W, Kong E, Meydani M (2009) Dietary polyphenols, inflammation, and cancer. Nutr Cancer 61(6):807–810. doi:10.1080/01635580903285098
Meydani M, Hasan ST (2010) Dietary polyphenols and obesity. Nutrients 2(7):737–751. doi:10.3390/nu2070737
Gocer H, Gulcin I (2011) Caffeic acid phenethyl ester (CAPE): correlation of structure and antioxidant properties. Int J Food Sci Nutr 62(8):821–825. doi:10.3109/09637486.2011.585963
Celik S, Erdogan S (2008) Caffeic acid phenethyl ester (CAPE) protects brain against oxidative stress and inflammation induced by diabetes in rats. Mol Cell Biochem 312(1–2):39–46. doi:10.1007/s11010-008-9719-3
Sawicka D, Car H, Borawska MH, Niklinski J (2012) The anticancer activity of propolis. Folia Histochem Cytobiol/Pol Acad Sci, Pol Histochem Cytochem Soc 50(1):25–37. doi:10.2478/18693
Kang LJ, Lee HB, Bae HJ, Lee SG (2010) Antidiabetic effect of propolis: reduction of expression of glucose-6-phosphatase through inhibition of Y279 and Y216 autophosphorylation of GSK-3alpha/beta in HepG2 cells. Phytother Res: PTR 24(10):1554–1561. doi:10.1002/ptr.3147
Chan GC, Cheung KW, Sze DM (2012) The immunomodulatory and anticancer properties of propolis. Clin Rev Allergy Immunol. doi:10.1007/s12016-012-8322-2
Weng YC, Chuang CF, Chuang ST, Chiu HL, Kuo YH, Su MJ (2012) KS370G, a synthetic caffeamide derivative, improves left ventricular hypertrophy and function in pressure-overload mice heart. Eur J Pharmacol 684(1–3):108–115. doi:10.1016/j.ejphar.2012.03.029
Weng YC, Chiu HL, Lin YC, Chi TC, Kuo YH, Su MJ (2010) Antihyperglycemic effect of a caffeamide derivative, KS370G, in normal and diabetic mice. J Agric Food Chem 58(18):10033–10038. doi:10.1021/jf1024246
Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F (1990) Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol 27(2--3):229--37
Lu DY, Leung YM, Cheung CW, Chen YR, Wong KL (2010) Glial cell line-derived neurotrophic factor induces cell migration and matrix metalloproteinase-13 expression in glioma cells. Biochem Pharmacol 80(8):1201–1209. doi:10.1016/j.bcp.2010.06.046
Chen JH, Huang SM, Chen CC, Tsai CF, Yeh WL, Chou SJ, Hsieh WT, Lu DY (2011) Ghrelin induces cell migration through GHS-R, CaMKII, AMPK, and NF-kappaB signaling pathway in glioma cells. J Cell Biochem 112(10):2931–2941. doi:10.1002/jcb.23209
Hanisch UK, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10(11):1387–1394. doi:10.1038/nn1997
Perry VH, Nicoll JA, Holmes C (2010) Microglia in neurodegenerative disease. Nat Rev Neurol 6(4):193–201. doi:10.1038/nrneurol.2010.17
Lehnardt S (2010) Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia 58(3):253–263. doi:10.1002/glia.20928
Kreutzberg GW (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19(8):312–318
Schwartz M (2003) Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab 23(4):385–394
Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK (2004) Role of microglia in central nervous system infections. Clin Microbiol Rev 17(4):942–964. doi:10.1128/CMR.17.4.942-964.2004, table of contents
Bal-Price A, Matthias A, Brown GC (2002) Stimulation of the NADPH oxidase in activated rat microglia removes nitric oxide but induces peroxynitrite production. J Neurochem 80(1):73–80
Gebicke-Haerter PJ (2001) Microglia in neurodegeneration: molecular aspects. Microsc Res Tech 54(1):47–58. doi:10.1002/jemt.1120
Seledtsov VI, Seledtsova GV (2012) A balance between tissue-destructive and tissue-protective immunities: a role of toll-like receptors in regulation of adaptive immunity. Immunobiology 217(4):430–435. doi:10.1016/j.imbio.2011.10.011
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122(3):787–795. doi:10.1172/JCI59643
Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P (2006) Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 211(6–8):487–501. doi:10.1016/j.imbio.2006.06.002
Martinez FO (2011) Regulators of macrophage activation. Eur J Immunol 41(6):1531–1534. doi:10.1002/eji.201141670
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3(1):23–35. doi:10.1038/nri978
Schwartz M, Butovsky O, Bruck W, Hanisch UK (2006) Microglial phenotype: is the commitment reversible? Trends Neurosci 29(2):68–74. doi:10.1016/j.tins.2005.12.005
Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P (2009) Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: Effects of oligomeric and fibrillar amyloid-beta. J Neuroimmunol 210(1–2):3–12. doi:10.1016/j.jneuroim.2009.02.003
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69. doi:10.1038/nrn2038
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155. doi:10.1002/glia.10161
Zhang Z, Zhang ZY, Wu Y, Schluesener HJ (2012) Lesional Accumulation of CD163(+) Macrophages/microglia in Rat Traumatic Brain Injury. Brain Res 1461:102–110. doi:10.1016/j.brainres.2012.04.038
Weis N, Weigert A, von Knethen A, Brune B (2009) Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell 20(5):1280–1288. doi:10.1091/mbc.E08-10-1005
Jeong GS, Lee DS, Li B, Lee HJ, Kim EC, Kim YC (2010) Effects of sappanchalcone on the cytoprotection and anti-inflammation via heme oxygenase-1 in human pulp and periodontal ligament cells. Eur J Pharmacol 644(1–3):230–237. doi:10.1016/j.ejphar.2010.06.059
Gabunia K, Ellison SP, Singh H, Datta P, Kelemen SE, Rizzo V, Autieri MV (2012) Interleukin-19 (IL-19) induces heme oxygenase-1 (HO-1) expression and decreases reactive oxygen species in human vascular smooth muscle cells. J Biol Chem 287(4):2477–2484. doi:10.1074/jbc.M111.312470
Tsoyi K, Kim HJ, Shin JS, Kim DH, Cho HJ, Lee SS, Ahn SK, Yun-Choi HS, Lee JH, Seo HG, Chang KC (2008) HO-1 and JAK-2/STAT-1 signals are involved in preferential inhibition of iNOS over COX-2 gene expression by newly synthesized tetrahydroisoquinoline alkaloid, CKD712, in cells activated with lipopolysacchride. Cell Signal 20(10):1839–1847. doi:10.1016/j.cellsig.2008.06.012
Vareille M, Rannou F, Thelier N, Glasser AL, de Sablet T, Martin C, Gobert AP (2008) Heme oxygenase-1 is a critical regulator of nitric oxide production in enterohemorrhagic Escherichia coli-infected human enterocytes. J Immunol 180(8):5720–5726
Lee TS, Chau LY (2002) Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8(3):240--6
Tai YH, Tsai RY, Lin SL, Yeh CC, Wang JJ, Tao PL, et al. (2009) Amitriptyline suppresses neuroinflammation-dependent interleukin-10-p38 mitogen-activated protein kinase-heme oxygenase-1 signaling pathway in chronic morphine-infused rats. Anesthesiology 110(6):1379-89
Alexander WS (2002) Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol 2(6):410–416. doi:10.1038/nri818
Baker BJ, Akhtar LN, Benveniste EN (2009) SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol 30(8):392–400. doi:10.1016/j.it.2009.07.001
Qin H, Roberts KL, Niyongere SA, Cong Y, Elson CO, Benveniste EN (2007) Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J Immunol 179(9):5966–5976
Dominguez E, Mauborgne A, Mallet J, Desclaux M, Pohl M (2010) SOCS3-mediated blockade of JAK/STAT3 signaling pathway reveals its major contribution to spinal cord neuroinflammation and mechanical allodynia after peripheral nerve injury. J Neurosci 30(16):5754–5766. doi:10.1523/JNEUROSCI.5007-09.2010
Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN (2008) Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol 181(5):3167–3176
Qin H, Yeh WI, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL, Fox TH 3rd, Park K, Harrington LE, Raman C, Benveniste EN (2012) Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A 109(13):5004–5009. doi:10.1073/pnas.1117218109
Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG (1998) AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J 334(1):177–187
Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF (1999) Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72(4):1707–1716
Kodiha M, Rassi JG, Brown CM, Stochaj U (2007) Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK-->ERK1/2 pathway. Am J Physiol Cell Physiol 293(5):C1427–C1436. doi:10.1152/ajpcell.00176.2007
Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282(41):30107–30119. doi:10.1074/jbc.M705325200
Jager S, Handschin C, St-Pierre J, Spiegelman BM (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A 104(29):12017–12022. doi:10.1073/pnas.0705070104
Zhang Y, Qiu J, Wang X, Xia M (2011) AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler Thromb Vasc Biol 31(12):2897–2908. doi:10.1161/ATVBAHA.111.237453
Okoshi R, Ozaki T, Yamamoto H, Ando K, Koida N, Ono S, Koda T, Kamijo T, Nakagawara A, Kizaki H (2008) Activation of AMP-activated protein kinase induces p53-dependent apoptotic cell death in response to energetic stress. J Biol Chem 283(7):3979–3987. doi:10.1074/jbc.M705232200
Zhang W, Zhang X, Wang H, Guo X, Li H, Wang Y, Xu X, Tan L, Mashek MT, Zhang C, Chen Y, Mashek DG, Foretz M, Zhu C, Zhou H, Liu X, Viollet B, Wu C, Huo Y (2012) AMP-activated protein kinase alpha1 protects against diet-induced insulin resistance and obesity. Diabetes. doi:10.2337/db11-1373
Sag D, Carling D, Stout RD, Suttles J (2008) Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol 181(12):8633–8641
Yang Z, Kahn BB, Shi H, Xue BZ (2010) Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem 285(25):19051–19059. doi:10.1074/jbc.M110.123620
Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, Gupta M, Chan L, Al-Omran M, Teoh H, Verma S (2010) Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. Am J Physiol Heart Circ Physiol 299(3):H656–H663. doi:10.1152/ajpheart.00115.2010
Wang S, Dale GL, Song P, Viollet B, Zou MH (2010) AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem 285(26):19976–19985. doi:10.1074/jbc.M110.102467
Nath N, Khan M, Rattan R, Mangalam A, Makkar RS, de Meester C, Bertrand L, Singh I, Chen Y, Viollet B, Giri S (2009) Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem Biophys Res Commun 386(1):16–20. doi:10.1016/j.bbrc.2009.05.106
Handy JA, Saxena NK, Fu P, Lin S, Mells JE, Gupta NA, Anania FA (2010) Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3). J Cell Biochem 110(5):1195–1207. doi:10.1002/jcb.22634
Acknowledgments
This work was supported by grants from the National Science Council (NSC 101-2320-B-039-048-MY2) and Taichung Tzu Chi General Hospital (TTCRD 101–03). The authors thank Ms Y. R. Chen for technical support.
Conflict of interest
The authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lu, DY., Huang, BR., Yeh, WL. et al. Anti-neuroinflammatory Effect of a Novel Caffeamide Derivative, KS370G, in Microglial cells. Mol Neurobiol 48, 863–874 (2013). https://doi.org/10.1007/s12035-013-8474-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8474-y