Abstract
Introduction
The kinetics of the transformation of ammonia and acid gases into components of PM2.5 has been examined. The interactions of existing aerosols and meteorology with the transformation mechanism have also been investigated. The specific objective was to discern the kinetics for the gas-to-particle conversion processes where the reactions of NH3 with H2SO4, HNO3, and HCl take place to form (NH4)2SO4, NH4NO3, and NH4Cl, respectively, in PM2.5.
Materials and methods
A Teflon-based outdoor environmental chamber facility (volume of 12.5 m3) with state-of-the-art instrumentation to monitor the concentration–time profiles of precursor gases, ozone, and aerosol and meteorological parameters was built to simulate photochemical reactions.
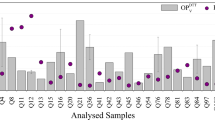
Results and discussion
The reaction rate constants of NH3 with H2SO4, HNO3, and HCl (i.e., k S, k N, and k Cl) were estimated as (1) k S = 2.68 × 10−4 (±1.38 × 10−4) m3/μmol/s, (2) k N = 1.59 × 10−4 (±8.97 × 10−5) m3/μmol/s, and (3) k Cl = 5.16 × 10−5 (±3.50 × 10−5) m3/μmol/s. The rate constants k S and k N showed significant day–night variations, whereas k Cl did not show any significant variation. The D/N (i.e., daytime/nighttime values) ratio was 1.3 for k S and 0.33 for k N. The significant role of temperature, solar radiation, and O3 concentration in the formation of (NH4)2SO4 was recognized from the correlation analysis of k S with these factors. The negative correlations of temperature with k N and k Cl indicate that the reactions for the formation of NH4NO3 and NH4Cl seem to be reversible under higher temperature due to their semivolatile nature. It was observed that the rate constants (k S, k N, and k Cl) showed a positive correlation with the initial PM2.5 levels in the chamber, suggesting that the existing surface of the aerosol could play a significant role in the formation of (NH4)2SO4, NH4NO3, and NH4Cl.
Conclusions
Therefore, this study recommends an intelligent control of primary aerosols and precursor gases (NO x , SO2, and NH3) for achieving reduction in PM2.5 levels.



Similar content being viewed by others
References
Aneja VP, Roelle PA, Murray GC, Southerland J, Erisman JW, Fowler D, Asman WAH, Patni N (2001) Atmospheric nitrogen compounds II: emissions, transport, transformation, deposition and assessment. Atmos Environ 35:1903–1911
Aneja VP, Schlesinger W, Erisman JW (2008) Farming pollution. Nature Geosci 1:409–411
Anttila T, Kerminen V-M, Kulmala M, Laaksonen A, O’Dowd CD (2004) Modelling the formation of organic particles in the atmosphere. Atmos Chem Phys 4:1071–1083
Baek BH, Aneja VP (2004) Measurement and analysis of the relationship between ammonia, acid gases, and fine particles in Eastern North Carolina. J Air and Waste Manag Assoc 54:623–633
Baek BH, Aneja VP, Tong Q (2004) Chemical coupling between ammonia, acid gases, and fine particles. Environ Pollut 129:89–98
Bassett M, Seinfeld JH (1984) Atmospheric equilibrium model of sulfate and nitrate aerosols—II. Particle size analysis. Atmos Environ 18:1163–1170
Behera SN, Sharma M (2010a) Investigating the potential role of ammonia in ion chemistry of fine particulate matter formation for an urban environment. Sci Total Environ 408:3569–3575
Behera SN, Sharma M (2010b) Reconstructing primary and secondary components of PM2.5 aerosol composition for an urban atmosphere. Aerosol Sci Technol 44:983–992
Behera SN, Sharma M (2011) Degradation of SO2, NO2 and NH3 leading to formation of secondary inorganic aerosols: an environmental chamber study. Atmos Environ 45:4015–4024
Behera SN, Sharma M, Dikshit O, Shukla SP (2011) GIS-based emission inventory, dispersion modeling, and assessment for source contributions of particulate matter in an urban environment. Water Air Soil Poll 218:423–436
Chow JC, Watson JG, Chen L-WA, Arnott WP, Moosmüller H, Fung K (2004) Equivalence of elemental carbon by thermal/optical reflectance and transmittance with different temperature protocols. Environ Sci Technol 38:4414–4422
Erisman JW, Vermetten AWM, Asman WAH, Waijers-Ijpelaan A, Slanina J (1988) Vertical distribution of gases and aerosols: the behavior of ammonia and related components in the lower atmosphere. Atmos Environ 22:1153–1160
Finlayson-Pitts BJ, Pitts JN (2006) Chemistry of the upper and lower atmosphere, 1st edn. Academic, San Diego, pp 92101–94495
Harrison RM, Kitto AN (1992) Estimation of the rate constant for the reaction of acid sulphate aerosol with NH3 gas from atmospheric measurements. J Atmos Chem 15:133–143
Ho KF, Lee SC, Chan CK, Yu JC, Chow JC, Yao XH (2003) Characterization of chemical species in PM2.5 and PM10 aerosols in Hong Kong. Atmos Environ 37:31–39
Holmes NS (2007) A review of particle formation events and growth in the atmosphere in the various environments and discussion of mechanistic implications. Atmos Environ 41:2183–2201
Jaoui M, Sexton KG, Kamens RM (2004) Reaction of α-cedrene with ozone: mechanism, gas and particulate products distribution. Atmos Environ 38:2709–2725
Lin YC, Cheng MT (2007) Evaluation of formation rates of NO2 to gaseous and particulate nitrate in the urban atmosphere. Atmos Environ 41:1903–1910
Lin YC, Cheng MT, Ting WY, Yeh CR (2006) Characteristics of gaseous HNO2, HNO3, NH3 and particulate ammonium nitrate in an urban city of Central Taiwan. Atmos Environ 40:4725–4733
Makar PA, Moran MD, Zheng Q, Cousineau S, Sassi M, Duhamel A, Besner M, Davignon D, Crevier LP, Bouchet VS (2009) Modelling the impacts of ammonia emissions reductions on North American air quality. Atmos Chem Phys 9:7183–7212
Martiän-Reviejo M, Wirtz K (2005) Is benzene a precursor for secondary organic aerosol? Environ Sci Technol 39:1045–1054
McMurry PH, Takano H, Anderson GR (1983) Study of the ammonia (gas)–sulphuric acid(aerosol) reaction rate. Environ Sci Technol 17:347–352
Mozurkewich M (1993) The dissociation-constant of ammonium-nitrate and its dependence on temperature, relative-humidity and particle-size. Atmos Environ 27:261–270
Odum JR, Jungkamp TPW, Griffin RJ, Flagan RC, Seinfeld JH (1997) The atmospheric aerosol-forming potential of whole gasoline vapor. Science 276:96–99
Orel AE, Seinfeld JH (1977) Nitrate formation in atmospheric aerosols. Environ Sci Technol 11:1000–1008
Ren X, Wang H, Shao K, Miao G, Tang X (2002) Determination and characteristics of OH radical in urban atmosphere in Beijing. Environ Sci (Huan Jing Ke Xue) 23(4):24–27 (in Chinese)
Renard JJ, Calidonna SE, Henley MV (2004) Fate of ammonia in the atmosphere—a review for applicability to hazardous releases. J Hazard Mater B108:29–60
Russell AG, McRae GJ, Cass GR (1983) Mathematical modeling of the formation and transport of ammonium nitrate aerosol. Atmos Environ 17:949–964
Sharma M, Maloo S (2005) Assessment and characterization of ambient air PM10 and PM2.5 in the city of Kanpur, India. Atmos Environ 39:6015–6026
Sharma M, Kishore S, Tripathi SN, Behera SN (2007) Role of atmospheric ammonia in the formation of inorganic secondary particulate matter: a study at Kanpur, India. J Atmos Chem 58:1–17
Tang IN (1980) On the equilibrium partial pressures of nitric acid and ammonia in the atmosphere. Atmos Environ 14:819–828
Trebs I, Meixner FX, Slanina J, Otjes R, Jongejan P, Andreae MO (2004) Real-time measurements of ammonia, acidic trace gases and water-soluble inorganic aerosol species at a rural site in the Amazon Basin. Atmos Chem Phys 4:967–987
Turpin BJ, Lim HJ (2001) Species contributions to PM2.5 mass concentrations: revisiting common assumptions for estimating organic mass. Aerosol Sci Technol 35:602–610
US Environmental Protection Agency (USEPA) (1998) Quality assurance guidance document 2.12. Monitoring PM2.5 in ambient air using designated reference or class I equivalent methods
US Environmental Protection Agency (USEPA) (1999a) Compendium of methods for the determination of inorganic compounds in ambient air, compendium method IO-3.1: selection, preparation and extraction of filter material. EPA/625/R–96/010a
US Environmental Protection Agency (USEPA) (1999b) Determination of the strong acidity of atmospheric fine-particle (<2.5 μm) using annular denuder technology. US Environmental Protection Agency, Atmospheric Research and Exposure Assessment Laboratory, Research Triangle Park, EPA/600/R-93/037
Wu Z, Hu M, Shao K, Slanina J (2009) Acidic gases, NH3 and secondary inorganic ions in PM10 during summertime in Beijing, China and their relation to air mass history. Chemosphere 76:1028–1035
Yu S, Dennis RL, Bhave PV, Eder BK (2004) Primary and secondary organic aerosols over the United States: estimates on the basis of observed organic carbon (OC) and elemental carbon (EC), and air quality modeled primary OC/EC ratios. Atmos Environ 38:5257–5268
Yu S, Dennis R, Roselle S, Nenes A, Walker J, Eder B, Schere K, Swall J, Robarge W (2005) An assessment of the ability of three-dimensional air quality models with current thermodynamic equilibrium models to predict aerosol NO −3 . J Geophys Res 110:D07S13. doi:10.1029/2004JD004718
Yu XY, Lee T, Ayres B, Kreidenweis SM, Malm W, JrJL C (2006) Loss of fine particle ammonium from denuded nylon filters. Atmos Environ 40:4797–4807
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Euripides Stephanou
Rights and permissions
About this article
Cite this article
Behera, S.N., Sharma, M. Transformation of atmospheric ammonia and acid gases into components of PM2.5: an environmental chamber study. Environ Sci Pollut Res 19, 1187–1197 (2012). https://doi.org/10.1007/s11356-011-0635-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0635-9