Abstract
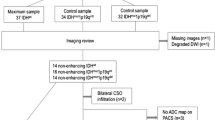
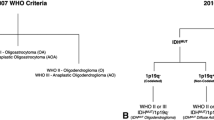
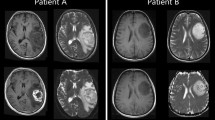
Purpose Apparent diffusion coefficient (ADC) describes water diffusion within tissues. Previous studies report a negative linear correlation between minimum ADC and tumour cellularity in different types of gliomas, but there are no studies in oligodendroglial tumours. This study evaluated the relationship between ADC and tumour cellularity in oligodendroglial tumours characterized by genotype. Methods ADC was assessed in 17 patients with known 1p/19q status: 3 grade II oligodendrogliomas (OII), 9 grade II oligoastrocytomas (OAII), 5 grade III oligoastrocytomas (OAIII). Regions of interest were placed on ADC maps around tumour margins to generate mean tumour ADC, and over minimum and maximum tumour ADC. Histopathology assessment of tumour cellularity determined minimum, maximum and mean cell density in serial stereotactic biopsies. Results 1p/19q loss was present in 2/3 OII, 5/9 OAII, 2/5 OAIII. Grade III tumours had higher maximum cell density than grade II tumours (17.2 vs. 10.57%: Mann Whitney U; P = 0.20). Oligoastrocytoma were more likely to have a lower minimum cell density than oligodendrogliomas (Mann Whitney U; P = 0.032). There was no relationship between cell density and genotype. There was no linear correlation between mean ADC and mean cell density (Spearman’s rho; r = 0.486: P = 0.438), minimum ADC and maximum cell density (Spearman’s rho; r = 0.158: P = 0.660), and maximum ADC and minimum cell density (Spearman’s rho; r = 0.039: P = 0.985). Conclusions In oligodendroglial tumours there is no relationship between quantitative assessment of cellularity and ADC. This may reflect differences in oligodendroglial tumour biology compared to other gliomas, although the composition of the extracellular matrix may influence ADC more than cellularity.






Similar content being viewed by others
References
Le Bihan D, Poupon C, Amadon A, Lethimonnier F (2006) Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging 24:478–488
Gupta RK, Cloughesy TF, Sinha U, Garakian J, Lazareff J, Rubino G, Rubino L, Becker DP, Vinters HV, Alger JR (2000) Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J Neurooncol 50:215–226
Sugahara T, Korogi Y, Kochi M, Ikushima I, Shigematu Y, Hirai T, Okuda T, Liang L, Ge Y, Komohara Y, Ushio Y, Takahashi M (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, Wakasa K, Yamada R (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:1081–1088
Chiang IC, Kuo YT, Lu CY, Yeung KW, Lin WC, Sheu FO, Liu GC (2004) Distinction between high-grade gliomas and solitary metastases using peritumoral 3-T magnetic resonance spectroscopy, diffusion, and perfusion imagings. Neuroradiology 46:619–627
Yang D, Korogi Y, Sugahara T, Kitajima M, Shigematsu Y, Liang L, Ushio Y, Takahashi M (2002) Cerebral gliomas: prospective comparison of multivoxel 2D chemical-shift imaging proton MR spectroscopy, echoplanar perfusion and diffusion-weighted MRI. Neuroradiology 44:656–666
Chenevert TL, Stegman LD, Taylor JM, Robertson PL, Greenberg HS, Rehemtulla A, Ross BD (2000) Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 92:2029–2036
Hein PA, Eskey CJ, Dunn JF, Hug EB (2004) Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25:201–209
Jager HR, Waldman AD, Benton C, Fox N, Rees J (2005) Differential chemosensitivity of tumor components in a malignant oligodendroglioma: assessment with diffusion-weighted, perfusion-weighted, and serial volumetric MR imaging. AJNR Am J Neuroradiol 26:274–278
Tozer DJ, Jager HR, Danchaivijitr N, Benton CE, Tofts PS, Rees JH, Waldman AD (2007) Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed 20:49–57
Megyesi JF, Kachur E, Lee DH, Zlatescu MC, Betensky RA, Forsyth PA, Okada Y, Sasaki H, Mizoguchi M, Louis DN, Cairncross JG (2004) Imaging correlates of molecular signatures in oligodendrogliomas. Clin Cancer Res 10:4303–4306
Jenkinson MD, du Plessis DG, Smith TS, Joyce KA, Warnke PC, Walker C (2006) Histological growth patterns and genotype in oligodendroglial tumours: correlation with MRI features. Brain 129:1884–1891
Jenkinson MD, Smith TS, Joyce KA, Fildes D, Broome J, du Plessis DG, Haylock B, Husband DJ, Warnke PC, Walker C (2006) Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology 48:703–713
Jenkinson MD, Smith TS, Brodbelt AR, Joyce KA, Warnke PC, Walker C (2007) Apparent diffusion coefficients in oligodendroglial tumours characterised by genotype. J Magn Reson Imaging 26:1405–1412
Walker C, du Plessis DG, Fildes D, Haylock B, Husband D, Jenkinson MD, Joyce KA, Broome J, Kopitski K, Prosser J, Smith T, Vinjamuri S, Warnke PC (2004) Correlation of molecular genetics with molecular and morphological imaging in gliomas with an oligodendroglial component. Clin Cancer Res 10:7182–7191
Walker C, du Plessis DG, Joyce KA, Fildes D, Gee A, Haylock B, Husband D, Smith T, Broome J, Warnke PC (2005) Molecular pathology and clinical characteristics of oligodendroglial neoplasms. Ann Neurol 57:855–865
Walker C, Haylock B, Husband D, Joyce KA, Fildes D, Jenkinson MD, Smith T, Broome J, du Plessis DG, Warnke PC (2006) Clinical use of genotype to predict chemosensitivity in oligodendroglial tumors. Neurology 66:1661–1667
Walker C, Haylock B, Husband D, Joyce KA, Fildes D, Jenkinson MD, Smith T, Broome J, Kopitzki K, du Plessis DG, Prosser J, Vinjamuri S, Warnke PC (2006) Genetic and metabolic predictors of chemosensitivity in oligodendroglial neoplasms. Br J Cancer 95:1424–1431
Louis DN, Ohgaki H, Wiestler OD, Cavanee WK (2007) WHO classification of tumours of the central nervous system. IARC Press, Lyon
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Gladson CL (1999) The extracellular matrix of gliomas: modulation of cell function. J Neuropathol Exp Neurol 58:1029–1040
Sadeghi N, Camby I, Goldman S, Gabius HJ, Baleriaux D, Salmon I, Decaesteckere C, Kiss R, Metens T (2003) Effect of hydrophilic components of the extracellular matrix on quantifiable diffusion-weighted imaging of human gliomas: preliminary results of correlating apparent diffusion coefficient values and hyaluronan expression level. AJR Am J Roentgenol 181:235–241
Beppu T, Inoue T, Shibata Y, Yamada N, Kurose A, Ogasawara K, Ogawa A, Kabasawa H (2005) Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neurol 63:56–61 (discussion 61)
Price SJ, Jena R, Burnet NG, Hutchinson PJ, Dean AF, Pena A, Pickard JD, Carpenter TA, Gillard JH (2006) Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 27:1969–1974
Stadlbauer A, Ganslandt O, Buslei R, Hammen T, Gruber S, Moser E, Buchfelder M, Salomonowitz E, Nimsky C (2006) Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 240:803–810
Acknowledgments
Supported by grants from The Walton Centre Neuroscience Fund, Clatterbridge Cancer Research Trust and The Royal Colleges of Edinburgh and Ireland.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jenkinson, M.D., du Plessis, D.G., Smith, T.S. et al. Cellularity and apparent diffusion coefficient in oligodendroglial tumours characterized by genotype. J Neurooncol 96, 385–392 (2010). https://doi.org/10.1007/s11060-009-9970-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-009-9970-9