Abstract
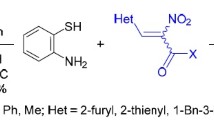
A series of 1,5-benzothiazepine derivatives were synthesized by the reaction of 1,5-benzothiazepine containing 2-phenoxy-quinoline with benzohydroximinoyl chlorides and hydrazonoyl chlorides at room temperature. The structures of these novel compounds were confirmed by spectrum, elemental, and X-ray crystallographic analysis.
Similar content being viewed by others
References
Jiang L, Wan X, Xu H, Bian J, Han W, Zhu K et al (2007) Effect of diltiazem and lidocaine on arterial pressure or heart rate and the quality of extubation in patients undergoing uvulopalato-pharyngoplasty. J Med Coll PLA 22: 230–233. doi:10.1016/S1000-1948(07)60046-X
Grandolini G, Perioli L, Ambrogi V (1999) Synthesis of some new 1,4-benzothiazine and 1,5-benzothiazepine tricyclic derivatives with structural analogy with TIBO and their screening for anti-HIV activity. Eur J Med Chem 34: 701–709. doi:10.1016/S0223-5234(99)00223-8
Richard S (2005) Vascular effects of calcium channel antagonists: new evidence. Drugs 65: 1–10. doi:10.2165/00003495-200565002-00002
Pei YZ, Michael JL, Owen DJ et al (2003) Efficient syntheses of benzothiazepines as antagonists for the mitochondrial sodium-calcium exchanger: potential therapeutics for type II diabetes. J Org Chem 68: 92–103. doi:10.1021/jo020446t
Kumari N, Saini RK, Joshi YC (2007) Synthesis of novel derivative of 1,5-benzothiazepines from 1,3-diketones and their antibacterial activity. Orient J Chem 23: 731–734
Zhu S (2004) Canadian multicenter clentiazem angina trial. S Chin J Card Dis 10: 72
Bariwal JB, Upadhyay KD, Manvar AT, Trivedi JC, Singh JS, Jain KS, Shah AK (2008) 1,5-Benzothiazepine, a versatile pharmacophore: a review. Eur J Med Chem 43: 2279–2290. doi:10.1016/j.ejmech.2008.05.035
Campiani G, Fiorini I, De Filippis MP, Ciani SM et al (1996) Cardiovascular characterization of pyrrolo[2,1-d][1,5]benzothiazepine derivatives binding selectively to the peripheral-type benzodiazepine receptor (PBR): from Dual PBR affinity and calcium antagonist activity to novel and selective calcium entry blockers. J Med Chem 39: 2922–2938. doi:10.1021/jm960162z
Ambrogi V, Grandolini G, Perioli L, Giusti L, Lucacchini A, Martini C (1995) Studies on annulated 1,4-benzothiazines and l,5-benzothiazepines. IX. Imidazo[2,1-d] [1,5]benzothiazepines: synthesis and in vitro benzodiazepine receptor affinity. Eur J Med Chem 30: 429–437. doi:10.1016/0223-5234(96)88253-5
Azad M, Munawar MA, Siddiqui HL (2007) Antimicrobial activity and synthesis of quinoline-based chalcones. J Appl Sci 7: 2485–2489
Chen YL, Fang KC, Sheu JY, Hsu SL, Tzeng CC (2001) Synthesis and antibacterial evaluation of certain quinolone derivatives. J Med Chem 44: 2374–2377. doi:10.1021/jm0100335
Tseng CH, Tzeng CC, Yang CL, Lu PJ, Chen HL, Li HY, Chuang YC, Yang CN, Chen YL (2010) Synthesis and antiproliferative evaluation of certain indeno[1,2-c]quinoline derivatives. J Med Chem 53: 6164–6179. doi:10.1021/jm1005447
Colin WW, Jonathan AK, Anthony GB, John EB, Marlene FC, Simon LC, Yaman G, Howard K, Roger MP, Pamela LP (2001) Synthesis and evaluation of cryptolepine analogues for their potential as new antimalarial agents. J Med Chem 44: 3187–3194. doi:10.1021/jm010929+
Zhang HZ, Kasibhatla S, Kuemmerle J, Kemnitzer W et al (2005) Discovery and structure–activity relationship of 3-aryl-5-aryl-1,2,4-oxadiazoles as a new series of apoptosis inducers and potential anticancer agents. J Med Chem 48: 5215–5223. doi:10.1021/jm050292k
Sakamoto T, Cullen MD, Hartman TL, Watson KM et al (2007) Synthesis and anti-HIV activity of new metabolically stable alkenyldiarylmethane non-nucleoside reverse transcriptase inhibitors incorporating N-methoxy imidoyl halide and 1,2,4-oxadiazole systems. J Med Chem 50: 3314–3321. doi:10.1021/jm070236e
Frizler M, Lohr F, Furtmann N, Klas J, Gutschow M (2011) Structural optimization of azadipeptide nitriles strongly increases association rates and allows the development of selective cathepsin inhibitors. J Med Chem 54: 396–400. doi:10.1021/jm101272p
Catarzi D, Colotta V, Varano F, Calabri FR, Filacchioni G, Galli A, Costagli C, Carla V (2004) Synthesis and biological evaluation of analogues of 7-chloro-4,5-dihy dro-4-oxo-8-(1,2,4-triazol-4-yl)-1,2,4-triazolo[1,5-a]quinoxaline-2-carboxylic acid (TQX-173) as novel selective AMPA receptor antagonists. J Med Chem 47: 262–272. doi:10.1021/jm030906q
Naito Y, Akahoshi F, Takeda S, Okada T, Kajii M, Nishimura H, Sugiura M, Fukaya C, Kagitani Y (1996) Synthesis and pharmacological activity of triazole derivatives inhibiting eosinophilia. J Med Chem 39: 3019–3029. doi:10.1021/jm9507993
Wadsworth HJ, Jenkins SM, Orlek BS, Cassidy F, Clark MSG, Brown F, Riley G J, Graves D, Hawkins J, Naylor CB (1992) Synthesis and muscarinic activities of quinuclidin-byltriazole and -tetrazole derivatives. J Med Chem 35: 1280–1290. doi:10.1021/jm00085a016
Burrell G, Evans JM, Hadley MS, Hicks F, Stemp G (1994) Benzopyran potassium channel activators related to cromakalim-heterocyclic amide replacements at position 4. Bioorg Med Chem Lett 4: 1285–1290. doi:10.1016/S0960-894X(01)80346-2
Stephens W, Field L (1959) A seven-membered heterocycle from o-aminobenzenethiol and chalcone. J Org Chem 24: 1576. doi:10.1021/jo01092a610
Pant S, Sharma P, Pant UC (2008) Synthesesand antimicrobial evaluation of 2-(2- Chlorophenyl)-4-(4-chlorophenyl/2-thienyl)-2,5-dihydro-8-substituted-1,5-benzothiazepines. Phosphorus Sulfur Silicon 183: 2974–2983. doi:10.1080/10426500802048920
Lu YC, Jin S, Xing QY (1988) Theoretical conformation analysis of 1,5-benzodiazepines and benzothiazepinds. J Mol Struct (Theochem) 167: 253–267. doi:10.1016/0166-1280(88)80230-6
Devi I, Baruah B, Bhuyan PJ (2006) α-cyclisation of tertiary amines: synthesis of some novel annelated quinolines via a three-component reaction under solvent-free conditions. Synlett 16: 2593–2596. doi:10.1055/s-2006-951477
Liu KC, Shelton BR, Howe RK (1980) A particularly convenient preparation of benzohydroximinoyl chlorides (nitrile oxide precursors). J Org Chem 45: 3916–3918. doi:10.1021/jo01307a039
Perrone MG, Santandrea E, Dell’Uomo N, Giannessi F, Milazzo FM, Sciarroni AF, Scilimati AF, Tortorella V (2005) Synthesis and biological evaluation of new clofibrate analogues as potential PPARa agonists. Eur J Med Chem 40: 143–154. doi:10.1016/j.ejmech.2004.09.018
Anderson WK, Jones AN (1984) Synthesis and evaluation of furan, thiophene, and azole bis[ (carbamoyloxy)methyl] derivatives as potential antineoplastic agents. J Med Chem 27: 1559–1565. doi:10.1021/jm00378a006
Xie ZF, Mo XX, Liu G, Liu FM (2008) Synthesis and crystal structure of 5-pyrazol-4,5-dihydropyrazoles derivatives. J Heterocycl Chem 45: 1485–1488. doi:10.1002/jhet.5570450539
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, ZQ., Liu, FM., Xu, F. et al. Synthesis of 1,5-benzothiazepine derivatives bearing 2-phenoxy-quinoline moiety via 1,3-diplolar cycloaddition reaction. Mol Divers 15, 963–970 (2011). https://doi.org/10.1007/s11030-011-9328-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-011-9328-z