Abstract
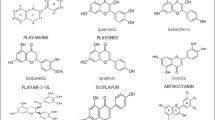
Doxorubicin is a widely used anthracycline anticancer agent. Its use may cause cardiomyopathy: in fact, the development of cumulative dose-related cardiotoxicity forms the major limitation of clinical doxorubicin use. We therefore searched for protective agents that combine iron-chelating and oxygen radical-scavenging properties. Moreover, any novel protector should not interfere with the cytostatic activity of doxorubicin. After extensive in vitro screening we found that flavonoids could serve this purpose. In particular 7-monohydroxyethylrutoside almost completely protected against the negative inotropic action of doxorubicin in the electrically paced mouse left atrium model. In vivo it gave full protection at 500 mg/kg intraperitoneally against the doxorubicin-induced ST-interval lengthening in the ECG. Moreover, this protector did not influence the antitumor effect of doxorubicin either in vitro using the human ovarian cell lines A2780 and OVCAR-3 and the human breast cancer cell line MCF-7 or in vivo in A2780 and OVCAR-3 subcutaneous xenografts in nude mice. Comparison of various iron chelators suggest that iron, in contrast to the general assumption, might not play a crucial role in the oxidative stress-induced toxicity of doxorubicin. Moreover, incubation of vascular endothelial cells with doxorubicin produced overexpression of adhesion molecules, which could be inhibited by 7-monohydroxyethylrutoside. From a study in human volunteers, we conclude that an intravenous dose of 1500 mg/m2 of 7-monohydroxyethylrutoside is feasible and is safe to be investigated as protection against doxorubicin-induced cardiotoxicity.
Similar content being viewed by others
Abbreviations
- ABAP:
-
azobisamidinopropane
- DMSO:
-
dimethyl sulfoxide
- DTNB:
-
5,5′-dithiobis(2-nitrobenzoic acid)
- GSH:
-
(reduced) glutathione
- HUVECs:
-
human umbilical cord vascular endothelial cells
- i.p.:
-
intraperitoneal(ly)
- i.v.:
-
intravenous(ly)
- LDH:
-
lactate dehydrogenase
- VCAM:
-
vascular cell adhesion molecule
References
Abou El Hassan MAI, Verheul HMW, et al. The new cardioprotector monohydroxyethylrutoside protects against doxorubicin-induced inflammatory effects in vitro. Br J Cancer. 2003;89:357–62.
Creighton AM, Hellmann K, Whitecross S. Antitumour activity in a series of bisketopiperazines. Nature. 1969;222:384–5.
de Celle T, Heeringa P, Strzelecka AE, Bast A, Smits JF, Janssen BJ. Sustained protective effects of 7-monohydroxyethylrutoside in an in vivo model of cardiac ischemia-reperfusion. Eur J Pharmacol. 2004;494:205–12.
Decorti G, Bartoli Klugmann F, Mallardi F, et al. Effects of ICRF 159 on adriamycin-induced cardiomyopathy in rats. Cancer Lett. 1983;19:77–83.
de Jong J, Schoofs PR, Snabilié AM, Bast A, van der Vijgh WJF. The role of biotransformation in anthracycline-induced cardiotoxicity in mice. J Pharmacol Exp Pharmacol. 1993;266:1312–20.
den Hartog GJM, Haenen GRRM, Boven E, van der Vijgh WJF, Bast A. Lecithinized copper, zinc-superoxide dismutase as a protector against doxorubicin-induced cardiotoxicity in mice. Toxicol Appl Pharmacol. 2004;194:180–8.
Doroshow JH. Anthracycline antibiotic-stimulated superoxide, hydrogen peroxide, and hydroxyl radical production by NADH dehydrogenase. Cancer Res. 1983;43:4543–51.
Frishman WH, Sung HM, Yee HC, et al. Cardiovascular toxicity with cancer chemotherapy. Curr Probl Cancer. 1997;21:301–60.
Goeptar AR, Te Koppele JM, Lamme EK, Piqué JM, Vermeulen NPE. Cytochrome P450 2B1-mediated one-electron reduction of adriamycin: a study with rat liver microsomes and purified enzymes. Mol Pharmacol. 1993;44:1267–77.
Holcenberg JS, Tutsch KD, Earhart RH, et al. Phase I study of ICRF-187 in paediatric cancer patients and comparison of its pharmacokinetics in children and adults. Cancer Treat Rep. 1986;70:703–9.
Hüsken BCP, de Jong J, Beekman B, Onderwater RCA, van der Vijgh WJF, Bast A. Modulation of the in vitro cardiotoxicity of doxorubicin by flavonoids. Cancer Chemother Pharmacol. 1995;37:55–62.
Jain D. Cardiotoxicity of doxorubicin and other anthracycline derivatives. J Nucl Cardiol. 2000;7:53–62.
Lefrak EA, Pitha J, Rosenheim S, Gotlieb JA. A clinicopathologic analysis of doxorubicin cardiotoxicity. Cancer. 1973;32:301–14.
Minotti G. Adriamycin-dependent release of iron from microsomal membranes. Arch Biochem Biophys. 1989;268:298–403.
Olson RD, Mushlin PS. Doxorubicin cardiotoxicity: analysis of the most prevailing hypotheses. FASEB J. 1990;4:3076–86.
Olson RD, Boerth RC, Gerber JG, Nies AS. Mechanism of adriamycin cardiotoxicity: evidence for oxidative stress. Life Sci. 1981;29:1393–401.
Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302.
Perkins WE, Schroeder RL, Carrano RA, Imondi AR. Effect of ICRF-187 on doxorubicin-induced myocardial effects in the mouse and guinea pig. Br J Cancer. 1982;46:662–7.
Schroterova L, Kaiserova H, Baliharova V, Velik J, Gersl V, Kvasnickova E. The effect of new lipophilic chelators on the activities of cytosolic reductases and P450 cytochromes involved in the metabolism of anthracycline antibiotics: studies in vitro. Physiol Res. 2004;53:683–91.
Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–7.
Tietze F. Enzymatic method for quantitative determination of nanogram amounts of total and oxidixed glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22.
van Acker FAA, van Acker SABE, Haenen GRMM, Bast A, van der Vijgh WJF. in vitro screening of antitumour agents for cardiotoxicity by means of isolated mouse left atria. Anticancer Res. 2000;20:4483–8.
van Acker SABE, Voest EE, Beems DB, et al. Cardioprotective properties of O-(beta-hydroxyethyl)-rutosides in doxorubicin-pretreated BALB/c mice. Cancer Res. 1993;53:4603–7.
van Acker SABE, Kramer K, Grimbergen JA, van den Berg D-J, van der Vijgh WJF, Bast A. Monohydroxyethylrutoside as protector against chronic doxorubicin-induced cardiotoxicity. Br J Pharmacol. 1995;115:1260–4.
van Acker SABE, van den Berg D-J, Tromp MNJL, et al. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med. 1996;20:331–42.
van Acker SABE, Boven E, Kuiper K, et al. Monohydroxyethylrutoside, a dose dependent cardioprotective agent, does not affect the antitumor activity of doxorubicin. Clin Cancer Res. 1997;3:1747–54.
van Acker SABE, Plemper van Balen G, van den Berg D-J, Bast A, van der Vijgh WJF. Influence of iron chelation on the antioxidant activity of flavonoids. Biochem Pharmacol. 1998;56:935–43.
Voest EE, van Acker SABE, van der Vijgh WJF, van Asbeck BS, Bast A. Comparison of different iron chelators as protective agents against acute doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol. 1994;26:1179–85.
Willems AM, Bruynzeel AM, Kedde MA, van Groeningen CJ, Bast A, van der Vijgh WJF. A phase I study of monohydroxyethylrutoside in healthy volunteers. Cancer Chemother Pharmacol. 2006;57:678–84.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bast, A., Kaiserová, H., den Hartog, G.J.M. et al. Protectors against doxorubicin-induced cardiotoxicity: Flavonoids. Cell Biol Toxicol 23, 39–47 (2007). https://doi.org/10.1007/s10565-006-0139-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-006-0139-4