Abstract
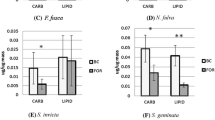
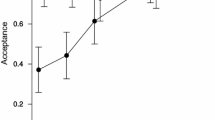
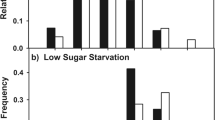
Disparities in liquid-feeding performances of major ant taxa have likely been important to resource partitioning among ants, to the nature and composition of ant partnerships with plants and sap-feeding trophobionts, and to ecological and evolutionary diversification of ant taxa. We measured performance volumetrically for individual workers of 77 ant species from lowland rain forests of Amazonia and Borneo and three key North American taxa. In trials with 9% sucrose solution, performances were strongly related to body size (and alitrunk length) and to proventricular structure at generic to subfamilial levels. Highly modified proventriculi were associated with disproportionately large load sizes in Formicinae and certain small-bodied Dolichoderinae. These same taxa also ingested liquids more rapidly during foraging than did similar-sized species with plesiomorphic proventriculi. Secondarily reduced foraging performances of several formicines likely reflect ecological or evolutionary trade-offs related to dietary specialization or anti-predator defenses. Across formicines and dolichoderines, performances differed by functional group. Relatively small loads and slow uptake characterized species tending trophobionts (mainly Hemiptera) day and night in large worker aggregations. Large loads and rapid uptake typified solitary, diurnal “leaf-foragers.” Intermediate feeding performances characterized a few species that both tended trophobionts in small aggregates and frequently foraged alone.



Similar content being viewed by others
References
Attygalle AB, Mutti A, Rohe W, Maschwitz U, Garbe W, Bestmann HJ (1998) Trail pheromone from the Pavan gland of the ant Dolichoderus thoracicus (Smith). Naturwissenschaften 85:275-277
Baroni Urbani C, de Andrade ML (1997) Pollen eating, storing and spitting by ants. Naturwissenschaften 84:256•258
Baroni-Urbani C, Bolton B, Ward PS (1992) The internal phylogeny of ants (Hymenoptera: Formicidae). Syst Entomol 17:301•329
Becerra JX, Venable DL (1989) Extrafloral nectaries: a defense against ant-Homoptera mutualisms? Oikos 55:276•280
Blakeman JP (1971) The chemical environment of the leaf surface in relation to growth of pathogenic fungi. In: Preece TF, Dickinson CH (eds) Ecology of leaf surface microorganisms. Academic, New York, pp 255•268
Blñ/4thgen N, Fiedler K (2002) Interactions between weaver ants Oecophylla smaragdina, homopterans, trees and lianas in an Australian rain forest canopy. J Anim Ecol 71:793•801
Blñ/4thgen N, Fiedler K (2003) Competition for composition: lessons from nectar-feeding ant communities. Ecology (in press)
Blñ/4thgen N, Verhaagh M, Goitia W, Jaffe K, Morawetz W, Barthlott W (2000) How plants shape the ant community in the Amazonian rainforest canopy: the key role of extrafloral nectaries and homopteran honeydew. Oecologia 125:229•240
Blñ/4thgen N, Gebauer G, Fiedler K (2003) Disentangling a rainforest food web using stable isotopes: dietary diversity in a species-rich ant community. Oecologia 137:426•435
Bolton B (1995) A new general catalog of the ants of the world. Harvard University Press, Cambridge, Mass.
Brñ/4hl CA, Gunsalam G, Linsenmair KE (1998) Stratification of ants (Hymenoptera, Formicidae) in a primary rain forest in Sabah, Borneo. J Trop Ecol 14:285•297
Chiotis M, Jermiin LS, Crozier RH (2000) A molecular framework for the phylogeny of the ant subfamily Dolichoderinae. Mol Phylogen Evol 17:108•116
Davidson DW (1988) Ecological studies of neotropical ant gardens. Ecology 69:1138•1152
Davidson DW (1997) The role of resource imbalances in the evolutionary ecology of tropical arboreal ants. Biol J Linn Soc 61:153•181
Davidson DW (1998) Resource discovery versus resource domination in ants: breaking the trade-off. Ecol Entomol 23:484•490
Davidson DW, McKey D (1993) The evolutionary ecology of symbiotic ant-plant relationships. J Hymenopt Res 2:13•83
Davidson DW, Cook SC, Snelling RR, Chua TH (2003) Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300:969•972
Dejean A, McKey D, Gibernau M, Belin M (2000) The arboreal ant mosaic in a Cameroonian rainforest (Hymenoptera: Formicidae). Sociobiology 35:403•423
Dohlen von C, Moran NA (1995) Molecular phylogeny of the Homoptera: a paraphyletic taxon. J Mol Evol 41:211•223
Eisner T (1957) A comparative morphological study of the proventriculus of ants (Hymenoptera: Formicidae). Bull Mus Comp Zool 116:441•490
Eisner T, Brown WL Jr (1958) The evolution and social significance of the ant proventriculus. Proc 10th Intl Congr Entomol 2:503•508
Eisner T, Wilson EO (1952) The morphology of the proventriculus of a formicine ant. Psyche 59:47•60
Fiala B (1990) Extrafloral nectaries vs ant-Homoptera mutualisms: a comment on Becerra and Venable. Oikos 59:281•282
Gil R et al (2003) The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc Natl Acad Sci USA 100:9388•9393
Graham P, Collett TS (2002) View-based navigation in insects: how wood ants (Formica rufa L.) look at and are guided by extended landmarks. J Exp Biol 205:2499-2509
Gronenberg W, Hölldobler B (1999) Morphologic representation of visual and antennal information in the ant brain. J Comp Neurol 412:229•240
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Heil M, Fiala B, Baumann B, Linsenmair KE (2000) Temporal, spatial and biotic variations in extrafloral nectar secretion by Macaranga tanarius. Funct Ecol 14:749-757
Heil M, Koch T, Hilpert A, Fiala B, Boland W, Linsenmair KE (2001) Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc Natl Acad Sci USA 98:1083-1088
Hölldobler B (1980) Canopy orientation: a new kind of orientation in ants. Science 210:86•88
Hölldobler B, Taylor RW (1983) A behavioral study of the primitive ant Nothomyrmecia macrops. Insect Soc 30:384•401
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge, Mass.
Hossaert-McKey M, Orivel J, Labeyrie E, Pascal L, Delabie JHC, Dejean A (2001) Differential associations with ants of three co-occurring extrafloral nectary-bearing plants. Ecoscience 8:325-335
Josens RB, Roces F (2000) Foraging in the ant Camponotus mus: nectar-intake rate and crop filling depend on colony starvation. J Insect Physiol 46:1103•1110
Josens RB, Farina WM, Roces F (1998) Nectar feeding by the ant Camponotus mus: intake rate and crop filling as a function of sucrose concentration. J Insect Physiol 44:579•585
Longino JT (2003) The Crematogaster (Hymenoptera, Formicidae, Myrmicinae) of Costa Rica. Zootaxa 15:1•150
Maschwitz U, Haenel H (1985) The migrating herdsman Dolichoderus cuspidatus and ant with a novel mode of life. Sociobiology 17:171•184
Maschwitz U, Maschwitz E (1974) Platzende Arbeiterinnen: eine neue Art der Feindabwehr bei sozialen Hautflñ/4glern. Oecologia 14:289•294
Mayr G (1862) Myrmecologische studien. Verh Zool-Bot Ges Wien 12:649•776
McKey D (1984) Interaction of the ant-plant Leonardoxa africana (Caesalpiniaceae) with its obligate inhabitants in a rainforest in Cameroon. Biotropica 16:81•99
Messina FJ (1981) Plant protection as a consequence of an ant membracid mutualisms: interactions on goldenrod Solidago sp. Ecology 62:1433•1440
Moran NA, Plague GR, Sandstrom JP, Wilcox JL (2003) A genomic perspective on nutrient provisioning by bacterial symbionts of insects. Proc Natl Acad Sci USA 100:14543•14548
Oliveira PS, Del-Claro K (2004) Multitrophic interactions in a neotropical savanna: ant-hemipteran systems, associated insect herbivores, and host plants. In: Burslem DFRP, Pinard MA, Hartley SE (eds) Biotic interactions in the tropics. Cambridge University Press, Cambridge
Pfeiffer M, Linsenmair KE (2000) Contributions to the life history of the Malaysian giant ant Camponotus gigas (Hymenoptera, Formicidae). Insect Soc 47:123•132
Read AF, Nee S (1995) Inference from binary comparative data. J Theor Biol 173:99•108
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223•225
SAS Institute (2001) JMP version 4.0.4. SAS institute, Cary, N.C.
Schmid-Hempel P, Kacelnik A, Houston AI (1985) Honeybees maximize efficiency by not filling their crop. Behav Ecol Sociobiol 17:61•66
Shattuck SO (1992a) Generic revision of the ant subfamily Dolichoderinae (Hymenoptera: Formicidae). Sociobiology 21:1•181
Shattuck SO (1992b) Higher classification of the ant subfamilies Aneuretinae, Dolichoderinae and Formicinae (Hymenoptera: Formicidae). Syst Ent 17:199•206
Shattuck SO (1992c) Review of the dolichoderine ant genus Iridomyrmex Mayr with descriptions of three new genera (Hymenoptera: Formicidae). J Aust Entomol Soc 31:13•18
Sokal RR, Rohlf FJ (1969) Biometry. Freeman, San Francisco
Swain RB (1980) Trophic competition among parabiotic ants. Insect Soc 27:377•390
Tjallingii WF (1995) Regulation of phloem sap feeding by aphids. In: Chapman RF, de Boer G (eds) Regulatory mechanisms in insect feeding. Chapman & Hall, New York, pp 120•209
Wheeler WM (1910) Ants. Columbia University Press, New York
Wilson EO (2003) Pheidole in the New World. Harvard University Press, Cambridge, Mass.
Yao I, Akimoto SI (2002) Flexibility in the composition and concentration of amino acids in honeydew of the drepanosiphid aphid Tuberculatus quercicola. Ecol Entomol 27:745•752
Yu DW, Davidson DW (1997) Experimental studies of species-specificity in Cecropia-ant relationships. Ecol Monogr 67:273•294
Acknowledgements
Studies were supported by the U. S. National Science Foundation (no. IBN-9707932 to D.W.D.). We thank Peru tm)s Instituto Nacional de Recursos Naturales (INRENA), and officials of the Parque Nacional Manu, Universidad Nacional Agraria de La Molina, the Universiti Brunei Darussalam, and the Brunei Museums, for granting or facilitating permissions to study and collect inside national parks. Specimens are vouchered in the Los Angeles County Natural History Museum and either (Peru) the Museo de Entomología of the Universidad National Agraria La Molina (or Museo de Historia Natural) or (Brunei) the Brunei Museums. For determinations of ant taxa, we thank E.O. Wilson (Pheidole), R.J. Kohout (Polyrhachis), and J.T. Longino (Crematogaster). D. Bramble and the reviewers commented helpfully on prior drafts, and E. King trained S. Cook in microscopy. M. Kilvington and T.H. Chua generously provide hospitality in Brunei. In 1974, W.L. Brown Jr, now deceased, first hinted to D.W.D. of a relationship between the sepalous proventriculus and the ecological and evolutionary success of formicines.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00442-005-1822-5
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Davidson, D.W., Cook, S.C. & Snelling, R.R. Liquid-feeding performances of ants (Formicidae): ecological and evolutionary implications. Oecologia 139, 255–266 (2004). https://doi.org/10.1007/s00442-004-1508-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1508-4