Abstract
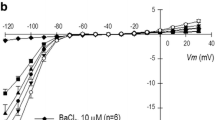
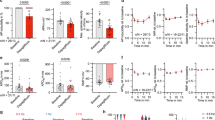
Previous work shows that transforming growth factor-β1 (TGF-β1) promotes several heart alterations, including atrial fibrillation (AF). In this work, we hypothesized that these effects might be associated with a potential modulation of Na+ and K+ channels. Atrial myocytes were cultured 1–2 days under either control conditions, or the presence of TGF-β1. Subsequently, Na+ (INa) and K+ (IK) currents were investigated under whole-cell patch-clamp conditions. Three K+ currents were isolated: inward rectifier (IKin), outward transitory (Ito), and outward sustained (IKsus). Interestingly, TGF-β1 decreased (50%) the densities of IKin and IKsus but not of Ito. In addition, the growth factor reduced by 80% the amount of INa available at −80 mV. This effect was due to a significant reduction (30%) in the maximum INa recruited at very negative potentials or Imax, as well as to an increased fraction of inactivated Na+ channels. The latter effect was, in turn, associated to a −7 mV shift in V1/2 of inactivation. TGF-β1 also reduced by 60% the maximum amount of intramembrane charge movement of Na+ channels or Qmax, but did not affect the corresponding voltage dependence of activation. This suggests that TGF-β1 promotes loss of Na+ channels from the plasma membrane. Moreover, TGF-β1 also reduced (50%) the expression of the principal subunit of Na+ channels, as indicated by western blot analysis. Thus, TGF-β1 inhibits the expression of Na+ channels, as well as the activity of K+ channels that give rise to IKsus and IKin. These results may contribute to explaining the previously observed proarrhythmic effects of TGF-β1.








Similar content being viewed by others
References
Armstrong CM (1981) Sodium channels and gating currents. Physiol Rev 61:644–683
Armstrong CM, Cota G (1990) Modification of sodium channel gating by lanthanum. Some effects that cannot be explained by surface charge theory. J Gen Physiol 96:1129–1140
Avila G, Medina IM, Jiménez E et al (2007) Transforming growth factor-β1 decreases cardiac muscle L-type Ca2+ current and charge movement by acting on the Cav1.2 mRNA. Am J Physiol Heart Circ Physiol 292:622–631
Balser JR (2001) The cardiac sodium channel: gating function and molecular pharmacology. J Mol Cell Cardiol 33:599–613
Bean BP, Rios E (1989) Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J Gen Physiol 94:65–93
Beffagna G, Occhi G, Nava A et al (2005) Regulatory mutations in transforming growth factor-beta3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res 65:366–373
Behfar A, Zingman LV, Hodgson DM et al (2002) Stem cell differentiation requires a paracrine pathway in the heart. FASEB J 16:1558–1566
Bers, D (2001) Excitation-Contraction Coupling and Cardiac Contractile Force, 2nd edn. Kluwer Academic Publishers 63–99
Brundel BJ, Van Gelder IC, Henning RH et al (2001) Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol 37:926–932
Brundel BJ, van Gelder IC, Henning RH et al (1999) Gene expression of proteins influencing the calcium homeostasis in patients with persistent and paroxysmal atrial fibrillation. Cardiovasc Res 42:443–454
Bujak M, Frangogiannis NG (2007) The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res 74:184–195
Burstein B, Nattel S (2008) Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51:802–809
Caballero R, de la Fuente MG, Gómez R et al (2010) In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol 55:2346–2354
Caffrey JM, Brown AM, Schneider MD (1989) Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: reversible suppression by transforming growth factor beta-1, an inhibitor of the myogenic pathway. J Neurosci 9:3443–3453
Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26:13–25
Chang H, Brown CW, Matzuk MM (2002) Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev 23:787–823
Deal KK, England SK, Tamkun MM (1996) Molecular physiology of cardiac potassium channels. Physiol Rev 76:49–67
Deynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–583
Er F, Larbig R, Ludwig A et al (2003) Dominant-negative suppression of HCN channels markedly reduces the native pacemaker current I(f) and undermines spontaneous beating of neonatal cardiomyocytes. Circulation 107:485–489
Fernández-Velasco M, Ruiz-Hurtado G, Hurtado O et al (2007) TNF-alpha downregulates transient outward potassium current in rat ventricular myocytes through iNOS overexpression and oxidant species generation. Am J Physiol Heart Circ Physiol 293:H238–H245
Field AC, Hill C, Lamb GD (1988) Asymmetric charge movement and calcium currents in ventricular myocytes of neonatal rat. J Physiol 406:277–297
Gaborit N, Steenman M, Lamirault G et al (2005) Human atrial ion channel and transporter subunit gene-expression remodeling associated with valvular heart disease and atrial fibrillation. Circulation 112:471–481
Gaspo R, Bosch RF, Bou-Abboud E et al (1997) Tachycardia-induced changes in Na+ current in a chronic dog model of atrial fibrillation. Circ Res 81:1045–1052
Gaspo R, Sun H, Fareh S et al (1999) Dihydropyridine and beta adrenergic receptor binding in dogs with tachycardia-induced atrial fibrillation. Cardiovasc Res 42:434–442
Josephson IR, Sperelakis N (1992) Kinetic and steady-state properties of Na+ channel and Ca2+ channel charge movements in ventricular myocytes of embryonic chick heart. J Gen Physiol 100:195–216
Kaczmarek LK, Bhattacharjee A, Desai R et al (2005) Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels. Hear Res 206:133–145
Lai LP, Su MJ, Lin JL et al (1999) Changes in the mRNA levels of delayed rectifier potassium channels in human atrial fibrillation. Cardiology 92:248–255
Li S, Li X, Zheng H et al (2008) Pro-oxidant effect of transforming growth factor-β1 mediates contractile dysfunction in rat ventricular myocytes. Cardiovasc Res 77:107–117
Li TS, Hayashi M, Ito H et al (2005) Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta- preprogrammed bone marrow stem cells. Circulation 111:2438–2445
Long CS (1996) Autocrine and paracrine regulation of myocardial cell growth in vitro. The TGFβ paradigm. Trends Cardiovasc Med 6:217–226
Mejia-Luna L, Avila G (2004) Ca2+ channel regulation by transforming growth factor-beta 1 and bone morphogenetic protein-2 in developing mice myotubes. J Physiol 559:41–54
Nakajima Y, Hokari S, Yamagishi T et al (2000) Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP). Anat Rec 258:119–127
Nattel S, Burstein B, Dobrev D (2008) Atrial remodeling and atrial fibrillation, mechanisms and implications. Circ arrhythmia electrophysiol 1:62–73
Nattel S, Maguy A, Le Bouter S et al (2007) Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87:425–456
Nerbonne JM, Kass RS (2005) Molecular physiology of cardiac repolarization. Physiol Rev 85:1205–1253
Ng CM, Cheng A, Myers LA et al (2004) TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 114:1586–1592
Perillan PR, Chen M, Potts EA et al (2002) Transforming growth factor-beta 1 regulates Kir2.3 inward rectifier K+ channels via phospholipase C and protein kinase C-delta in reactive astrocytes from adult rat brain. J Biol Chem 277:1974–1980
Petkova-Kirova PS, Gursoy E, Mehdi H et al (2006) Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol 290:H2098–H2107
Ramos-Mondragón R, Galindo CA, Avila G (2008) Role of TGF-beta on cardiac structural and electrical remodeling. Vasc Health Risk Manag 4:1289–1300
Robinson RB, Yu H, Chang F et al (1997) Developmental change in the voltage-dependence of the pacemaker current, if, in rat ventricle cells. Pflugers Arch 433:533–535
Schilling T, Eder C (2003) Effects of kinase inhibitors on TGF-beta induced upregulation of Kv1.3 K+ channels in brain macrophages. Pflugers Arch 447:312–315
Shih HT, Wathen MS, Marshall HB et al (1990) Dihydropyridine receptor gene expression is regulated by inhibitors of myogenesis and is relatively insensitive to denervation. J Clin Invest 85:781–789
Sossalla S, Kallmeyer B, Wagner S et al (2010) Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol 55:2330–2342
Sun ZQ, Ojamaa K, Nakamura TY et al (2001) Thyroid hormone increases pacemaker activity in rat neonatal atrial myocytes. J Mol Cell Cardiol 33:811–824
van Vliet P, de Boer TP, Sluijter JP et al (2007) Abstract 1074: Potassium inward rectifier expression is regulated by TGFß and BMP in human cardiomyocyte progenitor cells. Circulation 116:215
Van Wagoner DR, Pond AL, McCarthy PM et al (1997) Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res 80:772–781
Verheule S, Sato T, Everett T IV et al (2004) Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-β1. Circ Res 94:1458–1465
Wagner SR, Dybkova N, Rasenack EC et al (2006) Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 116:3127–3138
Wu MM, Gao PJ, Jiao S et al (2003) TGF-beta1 induces the expression of fast inactivating K+ (I(A)) channels in rat vascular myofibroblasts. Biochem Biophys Res Commun 301:17–23
Yue L, Feng J, Gaspo R et al (1997) Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 81:512–525
Yue L, Melnyk P, Gaspo R et al (1999) Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res 84:776–784
Zakon HH (1998) The effects of steroid hormones on electrical activity of excitable cells. Trends Neurosci 21:202–207
Acknowledgments
We would like to thank Mario Rodriguez and Esperanza Jimenez for the technical assistance. We also thank Dr. Gary Matthews for his generous gift of anti-pan Na+ channel antibody. This work was supported by a Conacyt grant to GA (56733).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramos-Mondragón, R., Vega, A.V. & Avila, G. Long-term modulation of Na+ and K+ channels by TGF-β1 in neonatal rat cardiac myocytes. Pflugers Arch - Eur J Physiol 461, 235–247 (2011). https://doi.org/10.1007/s00424-010-0912-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-010-0912-3