Abstract
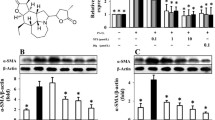
Nicotine exposure disrupts the parathyroid hormone-related protein (PTHrP)-driven alveolar epithelial-mesenchymal paracrine-signaling pathway, resulting in the transdifferentiation of pulmonary lipofibroblasts (LIFs) to myofibroblasts (MYFs), which seems to be central to altered pulmonary development and function in infants born to mothers who smoke during pregnancy. Modulation of PTHrP-driven signaling can almost completely prevent nicotine-induced LIF-to-MYF transdifferentiation. However, once this process has occurred, whether it can be reversed is not known. Our objective was to determine if nicotine-induced LIF-to-MYF transdifferentiation could be reversed by specifically targeting the PTHrP-mediated alveolar epithelial-mesenchymal paracrine signaling. WI38 cells, a human embryonic pulmonary fibroblast cell line, were initially treated with nicotine for 7 days and LIF-to-MYF transdifferentiation was confirmed by determining the downregulation of the key lipogenic marker, peroxisome proliferator-activated receptor γ (PPARγ) and upregulation of the key myogenic marker, α-smooth muscle actin (αSMA). Because downregulation of the PPARγ signaling pathway is the key determinant of LIF-to-MYF transdifferentiation, cells were treated with three agonists of this pathway, PTHrP, dibutryl cAMP (DBcAMP), or rosiglitazone (RGZ) for 7 days, and the expression of the PTHrP receptor, PPARγ, αSMA, and calponin was determined by Western analysis and immunohistochemistry. Simultaneously, fibroblast function was characterized by measuring their capacity to take up triglycerides. Nicotine-induced LIF-to-MYF transdifferentiation was almost completely reversed by treatment with RGZ, PTHrP, or DBcAMP, as determined by protein and functional assays. Using a specific molecular approach and targeting specific molecular intermediates in the PTHrP signaling pathway, to our knowledge, this for the first time, demonstrates the reversibility of nicotine-induced LIF-to-MYF transdifferentiation, suggesting not only the possibility of prevention but also the potential for reversal of nicotine-induced lung injury.








Similar content being viewed by others
References
Maritz GS (1988) Effect of maternal nicotine exposure on growth in vivo of lung tissue of neonatal rats. Biol Neonate 53:163–170
Walsh RA (1994) Effects of maternal smoking on adverse pregnancy outcomes: examination of the criteria of causation. Hum Biol 66:1059–1092
Chen MF, Kimizuka G, Wang NS (1987) Human fetal lung changes associated with maternal smoking during pregnancy. Pediatr Pulmonol 3:51–58
Collins MH, Moessinger AC, Kleinerman J, Bassi J, Rosso P, Collins AM, James LS, Blanc WA (1985) Fetal lung hypoplasia associated with maternal smoking: A morphometric analysis. Pediatr Res 19:408–412
Cunningham J, Dockery DW, Speizer FE (1994) Maternal smoking during pregnancy as a predictor of lung function in children. Am J Epidemiol 139:1139–1152
Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, Weiss ST, Speizer FE (1992) The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis 145:1129–1135
Sekhon HS, Keller JA, Proskocil BJ, Martin EL, Spindel ER (2002) Maternal nicotine exposure upregulates collagen gene expression in fetal monkey lung: association with (7 nicotinic acetylcholine receptors. Am J Respir Cell Mol Biol 26:31–41
Pierce RA, Nguyen NM (2002) Prenatal nicotine exposure and abnormal lung function. Am J Respir Cell Mol Biol 26:10–13
Rehan VK, Wang Y, Sugano S, Romero S, Chen X, Santos J, Khazanchi A, Torday JS (2005) Mechanism of nicotine-induced pulmonary fibroblast. Am J Physiol Lung Cell Mol Physiol 289:L667–L676
Torday JS, Rehan VK (2002) Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol 283:L130–L135
Schultz CJ, Torres E, Londos C, Torday JS (2002) Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol 283:L288–L296
Torday JS, Sun H, Wang L, Torres E, Sunday ME, Rubin LP (2002) Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am J Physiol Lung Cell Mol Physiol 282:L405–L410; erratum in Am J Physiol Lung Cell Mol Physiol (2002) 282(4) Section L
Torday JS, Hua J, Slavin R (1995) Metabolism and fate of neutral lipids of fetal lung fibroblast origin. Biochem Biophys Acta 1254:198–206
Bradford MA (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Rubin LP, Kovacs CS, De Paepe ME, Tsai SW, Torday JS, Kronenberg HM (2004) Arrested pulmonary alveolar cytodifferentiation and defective surfactant synthesis in mice missing the gene for parathyroid hormone-related protein. Dev Dyn 230:278–289
Torday JS, Torres E, Rehan VK (2003) The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation and repair. Pediatr Pathol Mol Med 22:189–207
Maritz GS, Dennis H (1998) Maternal nicotine exposure during gestation and lactation interferes with alveolar development in the neonatal lung. Reprod Fertil Dev 10:255–261
Hamilton BE, Martin JA, Sutton PD (2004) Births: preliminary data for 2003. Natl Vital Stat Rep 53:1–17
Pastrakuljic A, Schwartz R, Simone C, Derewlany LO, Knie B, Koren G (1998) Transplacental transfer and biotransformation studies of nicotine in the human placental cotyledon perfused in vitro. Life Sci 63:2333–2342
Luck W, Nau H, Hansen R, Steldinger R (1985) Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther 8:384–395
Szuts T, Olsson S, Lindquist NG, Ullberg S, Pilotti A, Enzell C (1978) Long-term fate of [14C]nicotine in the mouse: retention in the bronchi, melanin-containing tissues and urinary bladder wall. Toxicology 10:207–220
Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, Whitsett JA, Starcher BC, Spindel ER (2005) Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med 171:1032–1039
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by grants from the American Heart Association (0265127Y), the National Institutes of Health (HL075405 and HL55268), Philip Morris USA, Inc. and Philip Morris International (11108−02), and the Tobacco-Related Disease Research Program (14RT-0073 and 15IT-0250).
Presented in part at the Pediatric Academic Societies’ Meeting, San Francisco, CA, May 2006.
Rights and permissions
About this article
Cite this article
Rehan, V.K., Sakurai, R., Wang, Y. et al. Reversal of Nicotine-Induced Alveolar Lipofibroblast-to-Myofibroblast Transdifferentiation by Stimulants of Parathyroid Hormone-Related Protein Signaling. Lung 185, 151–159 (2007). https://doi.org/10.1007/s00408-007-9007-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-007-9007-0