Abstract
Despite the increasing interest in other classes of small RNAs, microRNAs (miRNAs) remain the most widely investigated and have been shown to play a role in a number of different processes in mammals. Many studies investigating miRNA function focus on the processing enzyme Dicer1, which is an RNAseIII protein essential for the biogenesis of active miRNAs through its cleavage of precursor RNA molecules. General deletion of Dicer1 in the mouse confirms that miRNAs are essential for development because embryos lacking Dicer1 fail to reach the end of gastrulation. Here we investigate the role of Dicer1 in urogenital tract development. We utilised a conditional allele of the Dicer1 gene and two Cre-expressing lines, driven by HoxB7 and Amhr2, to investigate the effect of Dicer1 deletion on both male and female reproductive tract development. Data presented here highlight an essential role for Dicer1 in the correct morphogenesis and function of the female reproductive tract and confirm recent findings that suggest Dicer1 is required for female fertility. In addition, HoxB7:Cre-mediated deletion in ureteric bud derivatives leads to a spectrum of anomalies in both males and females, including hydronephrotic kidneys and kidney parenchymal cysts. Male reproductive tract development, however, remains largely unaffected in the absence of Dicer1. Thus, Dicer1 is required for development of the female reproductive tract and also normal kidney morphogenesis.
Similar content being viewed by others
Introduction
The development and differentiation of the reproductive organs is an essential process for the survival of all species of higher organisms. In mice, the gonads arise at approximately 10 days post coitum (dpc) as a thickening on the ventromedial surface of the mesonephros, an embryonic excretory organ in some species. The mesonephros is a vital structure in mouse sexual development: it acts as a source of cells for subsequent development of the gonad and it also contains the reproductive tract anlagen of the male, the Wolffian (mesonephric) duct, and of the female, the Müllerian (paramesonephric) duct. The developing reproductive tracts have a sexually dimorphic fate during embryogenesis. Under the influence of testosterone produced by the developing testis, the Wolffian duct differentiates into the epididymis, vas deferens, and seminal vesicles in males. In female embryos, however, the Wolffian duct regresses. A branch of the Wolffian duct, the ureteric bud, forms at 10.5 dpc and is the developmental origin of the metanephric (permanent) kidney in both sexes. The bud stalk elongates to form the epithelial tube of the ureter as well as the epithelium lining the renal pelvis, while the bud branches recurrently to form the kidney collecting ducts, with branch tips inducing nephrogenic mesenchyme to undergo epithelial transition to form nephrons (proximal tubules and glomerular epithelia).
The Müllerian duct regresses in males, whereas in females the absence of testicular hormones results in the Müllerian duct differentiating to form the oviducts, uterus, cervix, and upper vagina of the reproductive tract (Kobayashi and Behringer 2003). The Müllerian duct forms at approximately 12.0 dpc in the mouse by a process of invagination and proliferation of cells derived originally from the coelomic epithelium (Guioli et al. 2007; Orvis and Behringer 2007). Two Wnt molecules, Wnt4 and Wnt9b, are known to be required for proper morphogenesis of the Müllerian duct (Carroll et al. 2005; Vainio et al. 1999) and the LIM-class homedomain protein, Lim1, is also required for development of the Müllerian duct epithelium (Kobayashi et al. 2004). Interestingly, these studies on Lim1 function in the Müllerian duct demonstrate that the Wolffian duct supports Müllerian duct elongation and maintenance. The sexually dimorphic fate of the Müllerian duct is regulated by the presence of the testicular hormone, anti-Müllerian hormone (Amh), in male embryos. Amh, a member of the TGFβ-like family of growth factors, is produced by Sertoli cells and signals to the developing ductal epithelial cells to initiate regression by apoptosis (Roberts et al. 1999). The type II receptor for Amh, Amhr2, is actually expressed in the mesenchymal cells that surround the Müllerian duct, suggesting that some paracrine mechanism, involving mesenchymal and epithelial cell interaction, initiates ductal regression. In male embryos loss of Amhr2 causes a failure of Müllerian duct regression, and mutant males exhibit persistent remnants of the female reproductive tract, which as a result renders the males infertile (Jamin et al. 2003). After birth, Amhr2 is also expressed in the granulosa cells of the ovary. A Cre recombinase line reproducing the endogenous expression profile of Amhr2 has been previously described and has been used to study gene function specifically in the developing Müllerian duct mesenchyme (Jamin et al. 2002) and adult ovary granulosa cells (Andreu-Vieyra et al. 2007; Jorgez et al. 2004) using conditionally targeted (floxed) mutant alleles.
Less is known about the molecular basis of sexually dimorphic development of the Wolffian duct, although androgen-dependent maturation in male embryos is thought to underlie this (Hannema and Hughes 2007; Welsh et al. 2007). Tissue-specific deletion of genes in the developing Wolffian duct is possible with the use of the HoxB7:Cre line, which expresses Cre recombinase from the earliest stages of Wolffian duct development at 9.5 dpc (Yu et al. 2002). The Hoxb7:Cre line has also been used to study the consequences of targeted gene deletion in the developing ureteric bud epithelium and its derivatives (Oxburgh et al. 2004; Rojek et al. 2006; Yu et al. 2002; Zhao et al. 2004).
Recently, attention has turned to post-transcriptional gene regulation as a means of regulating gene function during development. microRNAs (miRNAs) are small endogenous RNA molecules about 22 nucleotides (nt) in length that post-transcriptionally regulate gene expression and have been shown to play essential roles in the development of many multicellular organisms (Harfe et al. 2005; Harris et al. 2006; Yi et al. 2006). The RNAseIII enzymes Drosha and Dicer1 process these small regulatory RNAs, with the latter essential in the production of the mature and active form. Dicer1 recognises the stem loop structure of the precursor miRNAs (pre-miRNAs) and cleaves them to form the mature double-stranded miRNA. The active strand of this double-stranded miRNA is subsequently incorporated into the RNA-induced silencing complex (RISC) containing Argonaute proteins, which together direct gene silencing. This silencing occurs by the binding of the miRNA to the target mRNA, preventing its translation, or by signaling its degradation. The study of Dicer1 and its effects has indicated an essential role for this enzyme in developmental processes, with the loss of Dicer1 resulting in embryonic lethality by 7.5 dpc (Bernstein et al. 2003). Dicer1 is also highly conserved across species, further highlighting its biological importance.
Recent studies have demonstrated various roles for Dicer1 in the male and female germ line. Specifically, oocytes lacking Dicer1 fail to complete meiosis and also exhibit oocyte maturation and developmental impairment (Murchison et al. 2007). Females homozygous for a hypomorphic Dicer1 allele are infertile and failure to maintain pregnancy is attributed to impaired follicle growth and absence of corpora lutea (Otsuka et al. 2008). Deletion of Dicer1 in male germ cells also results in subfertility as a result of abnormal sperm development and defects in motility (Maatouk et al. 2008). Here we report an investigation of the role of Dicer1 in the development of the urogenital tract, including both male and female reproductive tracts, by utilising the Cre/loxP system to conditionally inactivate Dicer1 using the two aforementioned Cre deleter lines: Amhr2:Cre and HoxB7:Cre. Our data confirm a role for Dicer1 in the development of the female reproductive tract but not in the male reproductive tract. We also show that deletion of Dicer1 using HoxB7:Cre has no overt consequences for male reproductive tract function, but it does result in abnormal morphology and function of the kidney.
Materials and methods
Targeted ablation of Dicer1
The Cre recombination system was adopted to conditionally delete Dicer1 in specific tissues. Mice homozygous for a floxed allele of Dicer1, in which loxP sites flank the exons encoding the active RNAseIII domain of Dicer1 (Harfe et al. 2005), were bred with mice expressing Cre recombinase under the control of the Amhr2 locus (Amhr2:Cre, a gift from Richard Behringer) or the HoxB7 locus (HoxB7:Cre, purchased from The Jackson Laboratory). All mice generated by this breeding were of a mixed genetic background and genotyped as previously described (Harfe et al. 2005; Jamin et al. 2002; Yu et al. 2002).
Fertility testing
The fertility of the Amhr2Dicer1KO (Amhr2 Cre/+, Dicer1 flox/flox) and HoxB7Dicer1KO (HoxB7 Cre/+, Dicer1 flox/flox) mice, along with heterozygous controls, was assessed by mating to C57BL/6 J mice of known fertility. Experimental females [Amhr2Dicer1KO (n = 12), HoxB7Dicer1KO (n = 9)] were placed in matings at 8–12 weeks of age and checked for vaginal plugs the day after matings were set up to ensure normal mating behaviour. At 14.5 days post coitum (dpc) females were palpated to check for signs of pregnancy. If pregnant, females were opened and the number of fetuses recorded. Females were scored as infertile if three matings failed to result in pregnancy. Experimental males [Amhr2Dicer1KO (n = 22), HoxB7Dicer1KO (n = 11)] were mated to C57BL/6 J females. These females were killed at 14.5 dpc and the number of fetuses recorded. To check for signs of conception and assess the morphology of the female reproductive tract more closely in Amhr2Dicer1KO mice, a second group of experimental females (n = 3) and control heterozygous littermates [Dicer1 flox/+; Amhr2 Cre/+ (n = 3)] were mated to C57BL6/J stud males and females were sacrificed at 2.5 dpc. The oviducts were dissected by making a cut 2 mm below the uterotubical junction and flushed with culture media for collection of morulae. The number and quality of any existing embryos (and oocytes) were recorded. If healthy morulae were found, these were cultured ex vivo to blastocyst stage to ensure correct development. The ovaries, uterine horns, and oviducts (after flushing) were fixed in buffered formalin for further histologic analysis.
Histologic analysis
All animals were culled by cervical dislocation, and in the case of Amhr2Dicer1KO and HoxB7Dicer1KO females, the ovaries, oviducts, and uterine horns were carefully dissected and fixed in buffered formalin. In males, the vas deferens, seminal vesicle, epididymis, and bladder were dissected and fixed in buffered formalin. Kidneys were also dissected from HoxB7Dicer1KO animals of both sexes and fixed in buffered formalin. The testes from all males were separated and fixed in Bouin’s and then dehydrated in 70% ethanol after no more than 6 h. All tissues were paraffin-embedded postfixing. Serial sections were cut at 5 μm through the ovaries, oviducts, testes, and kidneys and step sections were taken through the uterine horns. Sections were stained with hematoxylin and eosin for histologic examination. Immunohistochemical analysis of adult Sertoli cells was performed using an anti-WT1 antibody (Dako M3561) as previously described (Rao et al. 2006). Anti-aquaporin-2 antibody, a marker of renal collecting ducts, was purchased from Chemicon (Chandlers Ford, Hampshire, UK).
Clinical chemistry
Mice from the HoxB7Dicer1KO colony were collected at 21 days post partum (dpp) and culled by barbiturate overdose to minimise thoracic and cervical damage. Blood was collected from the jugular vein and collected in a lithium heparinised tube. This sample was kept at room temperature with rocking prior to centrifugation. Biochemical analysis of plasma using an Olympus AU400 clinical chemistry analyser (Olympus Diagnostics, UK) was performed as previously described (Hough et al. 2002). In addition, full postmortem was performed on all the individuals in the litter and any abnormalities were recorded.
Expression analysis
Timed matings were set up between Amhr2 Cre/+ or HoxB7 Cre/+ females and male mice carrying the Gtrosa26 tm1Sor targeted mutation [Rosa26 Reporter (R26R)]. Embryos were harvested at a range of developmental stages from 9.5 to 17.5 dpc. Individuals were genotyped for the presence of Cre using the following primers: CreF: CGTACTGACGGTGGGAGAAT, CreR: CCCGGCAAAACAGGTAGTTA. In the case of both Amhr2 Cre/+ and HoxB7 Cre/+ individuals, the developing reproductive organs were dissected and stained for β-galactosidase (β-gal) as previously described (Warr et al. 2009; Whiting et al. 1991). In the case of the HoxB7 Cre/+ individuals, the kidneys and excretory system were also dissected and stained for β-gal. In addition, Amhr2 Cre/+ mice were collected at 28 dpp, reproductive tracts removed, and whole-mount staining for β-gal performed.
Results
Targeted deletion of Dicer1 in the embryonic female reproductive tract
To study the effect of Dicer1 deletion in the Müllerian duct and its derivatives, we utilised a floxed allele of the Dicer1 gene in which exons encoding most of the critical catalytic domain of Dicer1 have been flanked by loxP sites (Harfe et al. 2005). Anti-Müllerian hormone receptor type II (Amhr2) was previously identified in the developing reproductive tract mesenchyme of male and female embryos and was demonstrated to play an essential role in Müllerian duct regression in males (Mishina et al. 1996). A line of mice expressing Cre recombinase from the endogenous Amhr2 promoter has been used to target gene deletion to these embryonic structures (Jamin et al. 2002). To confirm the previously reported expression pattern of Amhr2:Cre, female carriers of the Cre transgene (Amhr2 Cre/+) were mated to R26R mice. Litters were collected at the ages of 12.5-17.5 dpc and also at 28 dpp. β-gal expression was identified in the Müllerian duct mesenchyme of both male and female embryos from 13.5 dpc (Fig. 1a, b). Strong staining was also observed in the ovary at 13.5 dpc. Later-stage embryos exhibited strong staining in the uterine horns and oviduct, as did adult females at 28 dpp, in which ovary staining was also observed (Fig. 1c). Staining was also observed in the embryonic and adult testis (Fig. 1b and data not shown). It should be noted that in some cases more widespread, “leaky” staining was also discovered in embryos from 9.5 dpc (data not shown). Individuals exhibiting such broad Cre expression would not be predicted to survive due to the known embryonic lethality associated with tissue-wide Dicer1 deletion (Bernstein et al. 2003).
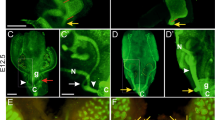
Deletion of Dicer1 in the developing female reproductive tract results in abnormalities of the oviduct and infertility. β-galactosidase expression is detected in the developing female reproductive tract (Müllerian duct, white arrow) of female (a) and male (b) embryos at 13.5 dpc. Strong β-galactosidase expression is also observed in the oviducts and uterine horns of adult females, with lower levels seen in the ovary (c). Histologic analysis of the ovaries from heterozygous controls (d) and Amhr2Dicer1KO females (e) reveals no apparent difference between the two. Both the control and the affected female ovaries display follicles in various stages of growth: primary follicles (1) characterised by a single layer of follicle epithelium; secondary follicles (2) displaying multilayers of granulosa cells surrounding the oocyte with supporting theca cells; and tertiary (3) or antral follicles characterised by clear antral space and increased thecal cell layers. Oviducts of heterozygous controls (f) displayed a uniformly smooth tubular structure, whereas the Amhr2Dicer1KO oviducts (g, h) were shorter and exhibited a thin, granular appearance. Oocytes could be observed trapped in the misshapen oviducts (g, white arrowhead). In extreme cases, cyst-like structures were identified in the oviducts of Amhr2Dicer1KO females (h, arrowheads). When the oviducts of the two groups were flushed at 2.5 dpc, the heterozygous controls produced healthy morulae (i), in addition to one unfertilised oocyte (i, white arrowhead) and one embryo with an extruded blastomere (i, black arrowhead). These were not considered to be abnormal. The Amhr2Dicer1KO oviducts contained debris with unfertilised oocytes and empty zona pellucidae (j). In one case a single embryo was identified but it was degenerate (j, white arrowhead)
To delete Dicer1 in the developing Müllerian duct, mice doubly heterozygous for the floxed Dicer1 allele (Dicer1 flox) and Amhr2 Cre were generated. These double heterozygous Amhr2 Cre/+, Dicer1 flox/+ females were mated with Dicer1 flox/flox males to produce Amhr2 Cre/+, Dicer1 flox/flox individuals (hereafter called Amhr2Dicer1KO), Amhr2 Cre/+, Dicer1 flox/+ (hereafter called heterozygous controls), and Amhr2 +/+, Dicer1 flox/flox and Amhr2 +/+, Dicer1 flox/+ littermates. Amhr2Dicer1KO animals were viable and externally indistinguishable from other genotypes. However, the expected Mendelian ratio was not observed, most likely because of occasional “leaky” expression of the Amhr2:Cre transgene described above (Table 1).
Amhr2Dicer1KO females are infertile
The reproductive tracts of Amhr2Dicer1KO males and females were examined for morphologic defects and compared with heterozygous controls at 8–12 weeks. Males and females from each class were killed and dissected transabdominally to reveal the reproductive system. In each case the terminal weights were recorded along with testis weights in males. Morphologically, the reproductive tracts of males and females appeared grossly normal to the naked eye and comparable to controls. There was no significant difference observed in the lengths or diameter of the Amhr2Dicer1KO uterine horns when compared with those of controls and natural variation was seen in both groups. No remnant of the Müllerian duct was observed in Amhr2Dicer1KO males, suggesting that Müllerian duct regression occurs normally in these mice. The fertility of Amhr2Dicer1KO males and females was then assessed using C57BL/6 J mice of proven fertility. Females (n = 12) were mated up to three times and males (n = 22) up to six times. Amhr2Dicer1KO females were infertile despite their mating behaviour being normal based on the presence of a copulatory plug (see Table 2). Amhr2Dicer1KO male mating behaviour and fertility appeared normal. As expected, heterozygous controls of both sexes were fertile (see Table 2).
Infertility of Amhr2Dicer1KO females is not caused by defects in ovulation but is determined by malformed oviducts
To investigate the possible causes of infertility in Amhr2Dicer1KO females, the ovaries and reproductive tracts were examined closely by histologic analysis. Initial analysis of section data revealed that the ovaries of the Amhr2Dicer1KO females were comparable to those of heterozygous controls. Examination of transverse ovary sections revealed a usual number of mature follicles in various stages of growth. Antral follicles containing oocytes were present, in addition to corpora lutea, indicating that ovulation occurs normally in Amhr2Dicer1KO females (Fig. 1d, e). Attention therefore turned to possible defects after ovulation and the maintenance of pregnancy. To investigate this further, matings between Amhr2Dicer1KO females and wild-type C56BL/6 J males were set up and checked for signs of a copulatory plug. Successfully plugged females were killed at 2.5 dpc, dissected, and their oviducts removed and placed in culture media. Detailed analysis of the Amhr2Dicer1KO oviducts showed morphologic abnormalities. All Amhr2Dicer1KO females examined (n = 3) at this time displayed oviducts that were reduced in size, with an uneven diameter compared with those of heterozygous littermate controls, and exhibited a granular appearance (Fig. 1g). Heterozygous controls (n = 4) displayed uniformly smooth oviducts that were tightly coiled (Fig. 1f). On closer examination (60× magnification), Amhr2Dicer1KO mice displayed small cysts along the length of their oviducts. The largest of these cysts appeared to occur at the uterotubical junction (Fig. 1h). One Amhr2Dicer1KO individual examined had anatomical abnormalities, with one oviduct displaying no clear tubular structure and the other containing large fluid-filled cysts (Fig. 1h and data not shown). Both Amhr2Dicer1KO and control oviducts were flushed with media to reveal signs of conception and assess the contents. The heterozygous control oviducts flushed easily, and of the four females examined, a total of 19 fertilised oocytes were found at various stages of development, from the four-cell stage to compact morulae (Fig. 1i). These were cultured in media overnight and most progressed successfully to the hatching blastocyst stage (data not shown). In contrast, the oviducts of the Amhr2Dicer1KO females were difficult to flush due to their irregular morphology. In one extreme case, the oviduct had poorly defined patency and we were unable to examine its contents. In some less extreme cases the oviducts of Amhr2Dicer1KO females had to be dissected down their length to collect their contents because flushing proved difficult, possibly due to occlusion. Persistent flushing of Amhr2Dicer1KO oviducts produced a lot of debris, with the majority of oocytes present in various stages of degradation (Fig. 1j). Evidence of previous ovulations was also observed, with the appearance of empty zona pellucidae, adding further weight to the idea that the oviducts may be occluded in some way. Oocytes could be seen through the thin transparent walls of the Amhr2Dicer1KO oviducts, collecting in the cyst-like structures, suggesting a possible defect in transport (Fig. 1g). Interestingly, we found only one example of possible fertilisation in the Amhr2Dicer1KO females but the embryo had degenerated (Fig. 1j).
Histologic analysis of Amhr2Dicer1KO reproductive tract morphology
The abnormal oviducts from the Amhr2Dicer1KO females were sectioned and histologic analysis confirmed the existence of oviductal cysts with a flattened mucosa (Fig. 2a–c). Amhr2Dicer1KO males at 8–12 weeks (n = 14) were fertile and had reproductive tracts and testes of normal appearance and average weight [0.12 ± 0.02 g (SD)] in comparison to heterozygous controls [0.11 ± 0.03 g (n = 4)]. Histologic analyses of two individuals showed that the majority of seminiferous tubules contained germ cells undergoing various stages of spermatogonial maturation (Fig. 2d, e). The presence of Sertoli cells in experimental and control samples was confirmed using immunohistochemical analysis of sections with an anti-WT1 (Wilms Tumour suppressor 1) antibody (data not shown).
Histologic examination of the reproductive tract of Amhr2Dicer1KO adult females revealed morphologic defects. Heterozygous control females (a) were compared with Amhr2Dicer1KO (b, c). Heterozygous control oviducts were uniform throughout their length and contained a single layer of tall cuboidal or tall columnar epithelial cells (a). The Amhr2Dicer1KO oviducts exhibited irregular morphology, with some containing large cyst-like structures (b). The oocytes can be seen trapped in these large cavities (arrows b and c). Histologic analysis of the testis from heterozygous control males showed seminiferous tubules undergoing normal spermatogenesis (d) Amhr2Dicer1KO male testes showed comparable morphology to controls (e). Scale bars = 100 μm
Targeted deletion of Dicer1 in the developing male reproductive tract
The HoxB7:Cre transgene is expressed in the developing Wolffian duct and its derivatives. This expression profile was confirmed by mating HoxB7 Cre/+ females to R26R males and staining for β-gal as described above. Strong β-gal expression was observed in the Wolffian duct and ureteric bud from 11.5 dpc (Fig. 3a). This expression persists in the Wolffian duct of both males and females at 13.5 dpc, and at this stage expression is observed in all the ureteric bud branches of the kidney. In adults, strong expression was detected in urothelia of the ureter and renal pelvis, and also in both medullary and cortical collecting ducts (Fig. 3b). In the male reproductive tract Cre expression was observed in the epithelium of the vas deferens, throughout the length of the epididymis, and also in the coagulating gland and seminal vesicle epithelium (data not shown).
HoxB7Dicer1KO mice have kidney defects. At 11.5 dpc prominent β-gal expression is observed throughout the length of the Wolffian duct (white arrow) and in the branching ureteric bud (white bracket) (a). Strong β-gal staining was observed in the adult ureter and renal pelvis and also in both medullary and cortical collecting ducts (b). HoxB7Dicer1KO mice (KO, red) of both sexes displayed a wide variety of kidney weights when compared to heterozygous controls (Het, blue) (c). Examination of HoxB7Dicer1KO (KO) kidneys revealed abnormalities including reduced size (d, right) and, in the most extreme cases, enlarged hydronephrotic kidneys (e). Heterozygous kidneys (Het) were smooth and dark red in comparison (d, left). Histologic analyses revealed healthy heterozygous kidneys with distinct cortical and medullary regions (f). Examination of HoxB7Dicer1KO kidneys confirmed a range of abnormalities with the most commonly observed being hydronephrosis, characterised by extreme compaction of the cortex and medulla (g). High magnification analysis of heterozygous controls showed normal cortex containing glomeruli (h), whereas high magnification in some HoxB7Dicer1KO cases revealed numerous cortical cysts (asterisks in i). Immunohistochemistry with an antibody to aquaporin-2, a collecting duct marker, detects normal, fine-calibre tubules (red) in the cortex of a heterozgous control kidney (j). In the HoxB7Dicer1KO kidney cortex, a subset of cysts and dilated tubules express aquaporin-2 (red), consistent with their origin in the ureteric bud/collecting duct lineage (k, l). c, cortex; m, medulla; g, glomerulus; pt, proximal tubule. Scale bars in d and e show millimetre divisions
To delete Dicer1 in the developing male reproductive tract, the same breeding scheme was used for the generation of Amhr2Dicer1KO mice. HoxB7 Cre/+, Dicer1 flox/flox individuals (hereafter called HoxB7Dicer1KO) and heterozygous controls (HoxB7 Cre/+, Dicer1 flox/+) were analysed. HoxB7Dicer1KO animals were viable, externally indistinguishable from heterozygous controls, and born in the expected Mendelian ratios (Table 1).
HoxB7Dicer1KO mice have overtly normal reproductive organs and are fertile
Morphologic assessment of HoxB7Dicer1KO mice was performed as described for the Amhr2Dicer1KO colony. At 8–12 weeks the reproductive organs of both male and female HoxB7Dicer1KO mice appeared normal. Testis weights of male mice (n = 14) were recorded and displayed an average value of 0.11 ± 0.02 g, which is normal for this age and comparable to that of controls [average = 0.11 ± 0.02 g (n = 5)]. Both male and female HoxB7Dicer1KO mice were fertility tested as described above and found to be fertile (see Table 2). Two of the 11 males tested failed to produce pregnant females. Histologic examination revealed that one had no obvious defects in the testes or reproductive tracts but one displayed hypoplastic seminal vesicles. This abnormality may be a low penetrance aspect of the phenotype of the HoxB7Dicer1KO genotype, but the number of cases was not enough to allow detailed further investigation.
Kidney abnormalities in HoxB7Dicer1KO mice
Kidneys of abnormal size were observed regularly in HoxB7Dicer1KO male (n = 14) and female (n = 21) mice. Kidney wet weights were recorded at 12 weeks of age and showed both increases and decreases versus age-matched controls (Fig. 3c). Furthermore, kidneys from HoxB7Dicer1KO mice of both sexes displayed a spectrum of gross morphologic anomalies (Fig. 3d, e) while heterozygous control mice of both sexes had kidney shapes that did not overtly differ from wild types (Fig. 3d). As assessed by histologic analyses, the most common kidney anomaly, present in 47% of mice, was hydronephrosis (a marked dilatation of the renal pelvis) (Fig. 3f, g). Both uni- and bilateral hydronephroses were found in HoxB7Dicer1KO kidneys of both sexes. In the most extreme examples of this phenotype, only a very thin rim of kidney cortex could be identified, and there was little medullary tissue (Fig. 3g). In others, however, cortical kidney parenchyma was relatively preserved with both glomeruli, proximal tubules, and collecting ducts present; in these samples, a subset of tubules were cystic (Fig. 3h, i). These cysts lacked a brush border, and some expressed the protein aquaporin-2 (Fig. 3j, k, l), consistent with these being derived from collecting ducts rather than proximal tubules (Chiu et al. 2006). Other HoxB7Dicer1KO kidneys at 12 weeks of age were not enlarged but sometimes had pale, pitted surfaces; in some of these, histology revealed foci of inflammation and scarring (data not shown). Hydronephrosis was also observed at 3 weeks of age (Fig. 4b), associated with ipsilateral hydroureter (Fig. 4d), but in only one kidney of the eight examined. The more common changes in HoxB7Dicer1KO kidneys at this age were cortical cysts (87.5% of those examined) and areas of dysplastic cortical tissue (75%) (Fig. 4e). These histologic changes were not seen in aged-matched controls (n = 3) (Fig. 4a, c). Blood plasma analyses of these four 3-week-old HoxB7Dicer1KO mice revealed a significant increase in urea and creatinine concentrations, results consistent with chronic kidney excretory failure; blood levels of albumin, however, were normal, suggesting that there was no gross leakage of protein by the diseased kidneys (Table 3).
Histologic analyses of mice at 3 weeks of age revealed renal abnormalities similar to those observed at later stages. At 3 weeks, the heterozygous controls displayed distinct cortical and medullary regions and a normal ureter (a, c). In one HoxB7Dicer1KO kidney, hydronephrosis was also observed at this early stage (b). This individual also displayed hydroureter (dilated ureteric lumen) with a ureteric smooth muscle coat that was attenuated in comparison to age-matched controls (d). In other cases, cortical cysts (asterisk, e) and areas of dysplasia (arrow, e) were also observed in the HoxB7Dicer1KO mice
Discussion
Several studies have demonstrated that loss of Dicer1, the gene encoding the main RNAseIII enzyme involved in the processing of microRNAs, has clear detrimental effects in many developmental processes. This study demonstrates that loss of Dicer1 in the developing female reproductive tract, under the control of Amhr2:Cre-mediated deletion, results in morphologic and functional defects in the reproductive tracts of female mice. In contrast, loss of Dicer1 in the developing male reproductive tract, in cells that have expressed the HoxB7:Cre transgene, results in no overt abnormalities before 3 months of age. However, severe defects in kidney morphology and function are observed in these animals. Data reported here concerning Amhr2Dicer1KO mice are consistent with two recent reports in which Dicer1 was deleted in the developing female reproductive tracts (Hong et al. 2008; Nagaraja et al. 2008). However, descriptions of a shortening of the uterine length in such animals differ from our observations of Amhr2Dicer1KO female mice. Interestingly, a hypomorphic allele of Dicer1 has been created using the Amhr2:Cre reporter line utilised in this study, and the ovaries exhibited absence of corpora lutea (Otsuka et al. 2008). Defects in hormone production were proposed to be the primary explanation for failure to maintain pregnancy in these mutants.
Careful analysis of the ovaries in Amhr2Dicer1KO females indicated that they were morphologically normal, suggesting that defects in ovary function were unlikely to be the primary cause of infertility in these mice. This is despite the expression of the Amhr2:Cre transgene in granulosa cells of the adult ovary. Histologic analysis of ovary sections clearly demonstrated oocytes in mature antral follicles and the presence of corpora lutea in all of the experimental females studied. Oviductal dissections of Amhr2Dicer1KO females after timed matings provided further evidence for normal ovary function because intact oocytes were discovered in the oviducts along with empty zona pellucidae. These latter observations suggest the occurrence of previous ovulations. However, we conclude that infertility in Amhr2Dicer1KO females is due to abnormal morphology of the oviducts. Experiments involving Amhr2Dicer1KO females mated to proven fertile C57BL/6 J males demonstrated an absence of morulae in the oviducts at 2.5 dpc, whereas oviducts of heterozygous controls contained healthy morulae at this stage. When morulae from heterozygous females were cultured ex vivo, they progressed as expected to the hatching blastocyst stage. The absence of morulae suggests that despite ovulation occurring in these Amhr2Dicer1KO females, the likelihood of fertilization is very low. This is possibly due to the presence of oviductal cysts that trap both oocytes and spermatozoa, preventing efficient fertilization. If fertilization does occur, there is a possibility that the resulting embryo will also be trapped in these cysts, preventing the normal passage of the embryo to the implantation site. As a consequence, any embryos that are formed are likely to degenerate within the oviduct. This eventuality was highlighted by the identification of a single degraded morula stage embryo in an Amhr2Dicer1KO female at 2.5 dpc (Fig. 1j).
Amhr2Dicer1KO males are fertile and comparable to controls at 8–12 weeks. Morphologic assessment of the male reproductive tract showed no defects and the testes of Amhr2Dicer1KO males were found to be morphologically normal with the expected average testis sizes and weights. Each Amhr2Dicer1KO male had testes that contained seminiferous tubules displaying normal spermatogenesis. Within these tubules germ cells and Sertoli cells were present. The expression of Amhr2:Cre in Sertoli cells of the developing embryo means that it will be important to reassess the fertility and testicular morphology of Amhr2Dicer1KO males at later time points. We also conclude that Dicer1 is dispensable for the appropriate regression of the developing female reproductive tract (Müllerian duct) in male embryos.
The Wolffian duct is the embryonic precursor of the mature male reproductive tract, comprising the vas deferens, seminal vesicle, and epididymis. Our studies using the HoxB7:Cre transgene to ablate Dicer1 in the developing male reproductive tract fail to reveal a role for this important enzyme, and thus miRNAs, in the development of these structures, at least in structures derived from cells that have expressed the transgene. Morphologic and histologic development of all these structures appears normal in the vast majority of HoxB7Dicer1KO males, and most males are fertile. It is unclear whether a low penetrance abnormality of the seminal vesicles is a reflection of a role for Dicer1 in the development of this structure. Moreover, infertility in a small number of males analysed may be secondary to renal failure. It is worth noting that some phenotypic variability between individuals is expected on a mixed genetic background.
In contrast, our studies do indicate a role for Dicer1 in renal tract morphogenesis. The predominant renal tract phenotype in the HoxB7Dicer1KO mice was hydronephrosis, accompanied by the absence of medullary tissues and the presence of cortical cysts. The Cre recombinase we used was expressed prominently in the ureteric bud and its renal tract derivatives in the HoxB7:Cre line, as evidenced by examination of the R26R reporter. The ureteric bud expresses several molecules such as the Ret receptor tyrosine kinase, Fras1 basement membrane protein, and Pax2 transcription factor, all essential for bud outgrowth (Clarke et al. 2006; Pitera et al. 2008). Bud-derived branches differentiate into collecting ducts that play a critical role in concentrating glomerular filtrate, and they express specific proteins, including HNF1b, a transcription factor that enhances differentiation and expression of various genes which downregulate growth (Gresh et al. 2004). Ablation of Dicer1 does not prevent bud initiation, but we speculate that Dicer1 may contribute to the regulation of expression of genes that affect collecting duct growth because of formation of cysts in HoxB7Dicer1KO kidneys. In addition to the ureteric bud and renal mesenchymal lineages, a third group of cells, mesenchyme originating in the tail bud, contributes to the renal tract (Brenner-Anantharam et al. 2007). These cells envelop differentiating urothelia and, from 15 dpc, they form ureteric smooth muscle, which actively propels urine from the renal pelvis into the bladder. These events require paracrine signaling by urothelial-derived sonic hedgehog (Yu et al. 2002). Failure of peristalsis leads to functional obstruction of urine flow and hydronephrosis (Caubit et al. 2008); thus, we speculate that the hydronephrosis observed in HoxB7Dicer1KO mice may result from incomplete ureter differentiation due to deregulation of gene expression in ureteric epithelia. Further work is now needed to unravel these possibilities. Our data complement recent reports that glomerular-specific ablation of Dicer1 also causes renal disease (Harvey et al. 2008; Shi et al. 2008). Loss of Dicer1 in glomerular podocytes, however, does not lead to hydronephrosis or collecting duct cysts but instead causes scarring of glomeruli.
In summary, the data described here demonstrate a role for Dicer1 in the developing female reproductive tract and in ureteric bud-derived renal tract tissues of both sexes. The phenotypic abnormalities observed as a result of Dicer1 depletion are most likely due to loss of miRNAs in these particular structures, although loss of other classes of small, noncoding RNA molecules cannot be discounted. The identity of the specific miRNAs responsible for these abnormalities is unknown, and determining which miRNAs are required for normal development of the female reproductive tract and kidneys, and what the targets of these miRNAs are, will form the basis of future research.
References
Andreu-Vieyra C, Chen R, Matzuk MM (2007) Effects of granulosa cell-specific deletion of Rb in Inha-alpha null female mice. Endocrinology 148:3837–3849
Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H et al (2003) Dicer is essential for mouse development. Nat Genet 35:215–217
Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT et al (2007) Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development 134:1967–1975
Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9:283–292
Caubit X, Lye CM, Martin E, Coré N, Long DA et al (2008) Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development 135:3301–3310
Chiu MG, Johnson TM, Woolf AS, Dahm-Vicker EM, Long DA et al (2006) Galectin-3 associates with the primary cilium and modulates cyst growth in congenital polycystic kidney disease. Am J Pathol 169:1925–1938
Clarke JC, Patel SR, Raymond RM Jr, Andrew S, Robinson BG et al (2006) Regulation of c-Ret in the developing kidney is responsive to Pax2 gene dosage. Hum Mol Genet 15:3420–3428
Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S et al (2004) A transcriptional network in polycystic kidney disease. EMBO J 23:1657–1668
Guioli S, Sekido R, Lovell-Badge R (2007) The origin of the Mullerian duct in chick and mouse. Dev Biol 302:389–398
Hannema SE, Hughes IA (2007) Regulation of Wolffian duct development. Horm Res 67:142–151
Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ (2005) The RNAseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA 102:10898–10903
Harris KS, Zhang Z, McManus MT, Harfe BD, Sun X (2006) Dicer function is essential for lung epithelium morphogenesis. Proc Natl Acad Sci USA 103:2208–2213
Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B et al (2008) Podocyte-specific deletion of Dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19:2150–2158
Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK (2008) Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 149:6207–6212
Hough TA, Nolan PM, Tsipouri V, Toye AA, Gray IC et al (2002) Novel phenotypes identified by plasma biochemical screening in the mouse. Mamm Genome 13:595–602
Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR (2002) Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32:408–410
Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR (2003) Genetic studies of the AMH/MIS signaling pathway for Mullerian duct regression. Mol Cell Endocrinol 211:15–19
Jorgez CJ, Klysik M, Jamin SP, Behringer RR, Matzuk MM (2004) Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol Endocrinol 18:953–967
Kobayashi A, Behringer RR (2003) Developmental genetics of the female reproductive tract in mammals. Nat Rev Genet 4:969–980
Kobayashi A, Shawlot W, Kania A, Behringer RR (2004) Requirement of Lim1 for female reproductive tract development. Development 131:539–549
Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD (2008) Dicer1 is required for differentiation of the mouse male germline. Biol Reprod 79:696–703
Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N et al (1996) Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev 10:2577–2587
Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ et al (2007) Critical roles for Dicer in the female germline. Genes Dev 21:682–693
Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R et al (2008) Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 22:2336–2352
Orvis GD, Behringer RR (2007) Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol 306:493–504
Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O et al (2008) Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest 118:1944–1954
Oxburgh L, Chu GC, Michael SK, Robertson EJ (2004) TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development 131:4593–4605
Pitera JE, Scambler PJ, Woolf AS (2008) Fras1, a basement-membrane-associated protein mutated in Fraser syndrome, mediates both the initiation of the mammalian kidney and the integrity of renal glomeruli. Hum Mol Genet 17:3953–3964
Rao MK, Pham J, Imam JS, MacLean JA, Murali D et al (2006) Tissue-specific RNAi reveals that WT1 expression in nurse cells controls germ cell survival and spermatogenesis. Genes Dev 20:147–152
Roberts LM, Hirokawa Y, Nachtigal MW, Ingraham HA (1999) Paracrine-mediated apoptosis in reproductive tract development. Dev Biol 208:110–122
Rojek A, Fuchtbauer EM, Kwon TH, Frokiaer J, Nielsen S (2006) Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci USA 103:6037–6042
Shi S, Yu L, Chiu C, Sun Y, Chen J et al (2008) Podocyte-selective deletion of Dicer induces proteinuria and glomerulosclerosis. J Am Soc Nephrol 19:2159–2169
Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397:405–409
Warr N, Siggers P, Bogani D, Brixey R, Pastorelli L et al (2009) Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol. doi:10.1016/j.ydbio.2008.11.023
Welsh M, Saunders PT, Sharpe RM (2007) The critical time window for androgen-dependent development of the Wolffian duct in the rat. Endocrinology 148:3185–3195
Whiting J, Marshall H, Cook M, Krumlauf R, Rigby PW et al (1991) Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev 5:2048–2059
Yi R, O’Carroll D, Pasolli HA, Zhang Z, Dietrich FS et al (2006) Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 38:356–362
Yu J, Carroll TJ, McMahon AP (2002) Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development 129:5301–5312
Zhao H, Kegg H, Grady S, Truong HT, Robinson ML et al (2004) Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276:403–415
Acknowledgments
The authors would particularly like to thank Nick Warr, Debora Bogani, Rachel Brixey, and Pam Siggers in the Sexual Development group at MGU, MRC Harwell for advice and support. We thank the staff of the Mary Lyon Centre (MLC) at MRC Harwell for support in animal husbandry, especially Lucie Vizor, Rose Kent, Dan Andrew, and Jackie Harrison. We thank Jim Humphrey, Dave Shipston, and Kate Vowell of the necropsy team of the MLC for assistance with dissections and the histology team for support in sectioning. We are grateful to Kan Pai Chiev for assistance with clinical chemistry. We thank Sue Rodger of the FESA team of the Mammalian Genetics Unit (MGU) for support in rederivation and analysis of preimplantation embryos. We are also grateful to Richard Behringer for supplying Amhr2:Cre mice. Support in statistics was kindly provided by Pete Underhill. Animal procedures used in this study were authorised by UK Home Office Project License PPL 30/2381. ASW acknowledges project grant support from Kidney Research UK and the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pastorelli, L.M., Wells, S., Fray, M. et al. Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mamm Genome 20, 140–151 (2009). https://doi.org/10.1007/s00335-008-9169-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-008-9169-y