Abstract
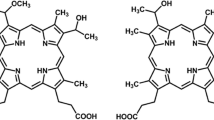
Current studies have indicated the utility of photodynamic therapy using porphyrins in the treatment of bacterial infections. Photoactivation of porphyrins results in the production of singlet oxygen (1O2) that damages biomolecules associated with cells and biofilms, e.g., proteins, polysaccharides, and DNA. The effect of a cationic porphryin on P. aeruginosa PAO1 biofilms was assessed by exposing static biofilms to 5,10,15,20-tetrakis(1-methyl-pyridino)-21H,23H-porphine, tetra-p-tosylate salt (TMP) followed by irradiation. Biofilms were visualized using confocal laser scanning microscopy (CLSM) and cell viability determined using the LIVE/DEAD BacLight viability assay and standard plate counts. At a concentration of 100 μM TMP, there was substantial killing of P. aeruginosa PAO1 wild-type and pqsA mutant biofilms with little disruption of the biofilm matrix or structure. Exposure to 225 μM TMP resulted in almost complete killing as well as the detachment of wild-type PAO1 biofilms. In contrast, pqsA mutant biofilms that contain less extracellular DNA remained intact. Standard plate counts of cells recovered from attached biofilms revealed a 4.1-log10 and a 3.9-log10 reduction in viable cells of wild-type PAO1 and pqsA mutant strains, respectively. Our results suggest that the action of photoactivated TMP on P. aeruginosa biofilms is two-fold: direct killing of individual cells within biofilms and detachment of the biofilm from the substratum. There was no evidence of porphyrin toxicity in the absence of light; however, biofilms pretreated with TMP without photoactivation were substantially more sensitive to tobramycin than untreated biofilms.




Similar content being viewed by others
References
Allesen-Holm M, Barken KB, Yang L et al (2006) A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol Microbiol 59:1114–1128
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322
Costerton JW (2001) Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol 9:50–52
Craven RC, Montie TC (1983) Chemotaxis of Pseudomonas aeruginosa: Involvement of Methylation. J Bacteriol 154:780–786
D’Argenio DA, Calfee MW, Rainey PB et al (2002) Autolysis and autoagreggation of Pseudomonas aeruginosa colony morphology mutants. J Bacteriol 184:6481–6489
Di Poto A, Sbarra MS, Provenza G et al (2009) The effect of photodynamic treatment combined with antiobiotic action or host defence mechanism on Staphylococcus aureus biofilms. Biomaterials 30:3158–3166
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Donnelly RF, McCarron PA, Cassidy CM et al (2007) Delivery of photosensitisers and light through mucus: investigations into the potential use of photodynamic therapy for treatment of Pseudomonas aeruginosa cystic fibrosis pulmonary infection. J Control Release 117:217–226
Freidman L, Kolter R (2004) Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol 51:675–690
Govan JR, Deretic V (1996) Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev 60:539–574
Hamblin MR, Hasan T (2004) Photodynamic therapy: a new antimicrobial approach to infectious disease. Photochem Photobiol Sci 3:436–450
Kelly JM, Murphy MJ (1985) A comparative study of the interaction of 5,10,15,20-tetrakis(N-methylpyridinum-4-yl)porphyrin and its zinc complex with DNA using fluorescence spectroscopy and topoisomerisation. Nucleic Acids Res 13:167–184
Matsukawa M, Greenberg EP (2004) Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J Bacteriol 186:4449–4456
Nemoto K, Hirota K, Mukarami K et al (2003) Effect of Varidase (streptodornase) on biofilm formed by Pseudomonas aeruginosa. Chemotherapy 49:121–125
Nickel JC, Ruseska I, Wright JB et al (1985) Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary tract catheter. Antimicrob Agents Chemother 27:619–624
Pasternack RF, Gibbs EJ (1996) Porphyrin and metalloporphyrin interactions with nucleic acids. Met Ions Biol Syst 33:367–397
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Speer AG, Cotton PB, Rode J et al (1988) Biliary stent blockage with bacterial biofilm. A light and electron microscopy study. Ann Intern Med 108:546–553
van Delden C (2004) Virulence factors in Pseudomonas aeruginosa. In: Ramos J-L (ed) Pseudomonas: virulence and gene regulation. Kluwer Academic/Plenum Publishers, New York, pp 3–45
van Delden C, Iglewski BH (1998) Cell-to-cell signaling and Pseudomonas aeruginosa infections. Infect Dis 4:551–560
Wainwright M (1998) Photodynamic antimicrobial chemotherapy (PACT). J Antimicrob Chemother 42:13–28
Wainwright M (2009) Photoantimicrobials—So what’s stopping us? Photodiag Photodyn Ther 6:167–169
Whitchurch CB, Tolker-Nielsen T, Ragas PC et al (2002) Extracellular DNA is required for bacterial biofilm formation. Science 295:1487
Acknowledgments
We would like to thank Dr. Mark Masthay for his technical assistance in the determination of light intensity and Dr. W. Dietz Bauer for his input on the manuscript. This study was supported by “Merck Institute for Science Education.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Tracy L. Collins and Elizabeth A. Markus contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Collins, T.L., Markus, E.A., Hassett, D.J. et al. The Effect of a Cationic Porphyrin on Pseudomonas aeruginosa Biofilms. Curr Microbiol 61, 411–416 (2010). https://doi.org/10.1007/s00284-010-9629-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9629-y