Abstract
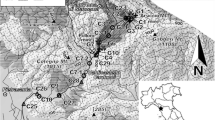
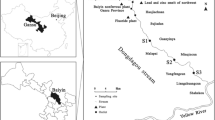
Transport and sediment–water partitioning of trace metals (Cr, Co, Fe, Pb, Cu, Ni, Zn, Cd) in acid mine drainage were studied in two creeks in the Kwangyang Au–Ag mine area, southern part of Korea. Chemical analysis of stream waters and the weak acid (0.1 N HCl) extraction, strong acid (HF–HNO3–HClO4) extraction, and sequential extraction of stream sediments were performed. Heavy metal pollution of sediments was higher in Chonam-ri creek than in Sagok-ri creek, because there is a larger source of base metal sulfides in the ores and waste dump upstream of Chonam-ri creek. The sediment–water distribution coefficients (K d) for metals in both creeks were dependent on the water pH and decreased in the order Pb ≈ Al > Cu > Mn > Zn > Co > Ni ≈ Cd. K d values for Al, Cu and Zn were very sensitive to changes in pH. The results of sequential extraction indicated that among non-residual fractions, Fe–Mn oxides are most important for retaining trace metals in the sediments. Therefore, the precipitation of Fe(–Mn) oxides due to pH increase in downstream sites plays an important role in regulating the concentrations of dissolved trace metals in both creeks. For Al, Co, Cu, Mn, Pb and Zn, the metal concentrations determined by 0.1 N HCl extraction (Korean Standard Method for Soil Pollution) were almost identical to the cumulative concentrations determined for the first three weakly-bound fractions (exchangeable + bound to carbonates + bound to Fe–Mn oxides) in the sequential extraction procedure. This suggests that 0.1 N HCl extraction can be effectively used to assess the environmentally available and/or bioavailable forms of trace metals in natural stream sediments.









Similar content being viewed by others
References
Anderson PR, Christensen TH (1988) Distribution coefficients of Cd, Co, Ni and Zn in soils. J Soil Sci 39:15–22
Balistrieri LS, Murray JW (1986) The surface chemistry of sediments from the Panama Basin: the influence of Mn oxides on metal adsorption. Geochim Cosmochim Acta 50:2235–2243
Benjamin MM, Leckie JO (1981) Multiple-site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J Colloid Interface Sci 79:209–221
Buchter B, Davidoff B, Amacher MC, Hinz C, Iskander IK, Selim HM (1989) Correlation of Freundlich K d and N retention parameters with soils and elements. Soil Sci 148:370–379
Campbell PGC, Lewis AG, Chapman PM (1988) Biologically available metals in sediments. National Research Council of Canada Associate Committee on Scientific Criteria for Environmental Quality. Publication no. NRCC 27694. NRCC, Ottawa
Dassenakis M, Degaita A, Scoullos M (1995) Trace metals in sediments of a Mediterranean estuary affected by human activities (Acheloos river estuary, Greece). Sci Total Environ 168:19–31
Datta DK, Subramanian V (1998) Distribution and fractionation of heavy metals in the surface sediments of the Ganges–Brahmaputra–Meghna river system in the Bengal basin. Environ Geol 36:93–101
Duinker JC, van Eck GTM, Nolting RF (1974) On the behaviour of copper, zinc, iron and manganese, and evidence for mobilization processes in the Dutch Wadden Sea. Neth J Sea Res 8:214–239
Dzombak DA, Morel FMM (1990) Surface complexation modeling; hydrous ferric oxide. Wiley Interscience, New York
Fiszman M, Pfeiffer WC, Drude de Lacerda L (1984) Comparison of methods used for extraction and geochemical distribution of heavy metals in bottom sediments from Sepetiba Bay, RJ (Brazil). Environ Technol Lett 5:567–575
Galán E, Gómez-Ariza JL, González I, Fernández-Caliani JC, Morales E, Giráldez I (2003) Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl Geochem 18:409–421
Hamilton RS, Revitt DM, Warren RS (1984) Levels and physico-chemical associations of Cd, Cu, Pb and Zn in road sediments. Sci Total Environ 33:59–74
Harrison RMD, Laxen PH and Wilson SJ (1981) Chemical association of Pb, Cd, Cu and Zn in street dust and road side soils. Environ Sci Technol 15:1378–1383
Henderson PJ, McMartin I, Hall GE, Percival JB, Walker DA (1998) The chemical and physical characteristics of heavy metals in humus and till in the vicinity of the base metal smelter at Flin Flon, Manitoba, Canada. Environ Geol 34:39–58
Jonasson IR (1977) Geochemsitry of sediment/water interactions of metals, including observations on availability. In: Shear H, Watson AEP (eds) The fluvial transport of sediment-associated nutrients and contaminants. IJC/PLUARG, Windsor
Kelly KD, Taylor CD (1997) Environmental geochemistry of shale-hosted Ag–Pb–Zn massive sulfide deposits in northwest Alaska: natural background concentrations of metals in water from mineralized areas. Appl Geochem 12:397–409
Kwon SH (1997) Mineralogical and geochemical studies of hydrothermal Au–Ag mineralization in the Kwangyang area, Korea. PhD Thesis, Korea University
Leckie JO, Tripathi VS (1985) Effect of geochemical parameters on the distribution coefficient K d. In: Proceedings of International Conference on Heavy Metals Environment. Athens, pp 369–371
Lee SZ, Allen HE, Huang CP, Sparks DL, Sanders PF, Peijnenburg WJGM (1996) Predicting soil–water partitioning coefficients for cadmium. Environ Sci Technol 30:3418–3424
Lee G, Bigham JM, Faure G (2002) Removal of trace metals by coprecipitation with Fe, Al and Mn from natural waters contaminated with acid mine drainage in the Ducktown Mining District, Tennessee. Appl Geochem 17:569–581
Li X, Thornton I (2001) Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Appl Geochem 16:1693–1706
Li X, Coles BJ, Ramsey MH, Thornton I (1995) Sequential extraction of soils for multielement analysis by ICP-AES. Chem Geol 124:109–123
Lion LW, Altmann RS, Leckie JO (1982) Trace metal adsorption characteristics of estuarine particulate matter: evaluation of contributions of Fe/Mn oxide and organic surface coatings. Environ Sci Technol 16:660–666
MOE (1997) Standard method for test of soil pollution. Ministry of Environment of the Republic of Korea, Seoul
Paul AC, Pillai KC (1983) Trace metals in a tropical river environment—distribution. Water Air Soil Poll 19:63–73
Plumlee GS, Smith KS, Ficklin WH (1994) Geoenvironmental models of mineral deposits and geology-based mineral-environmental assessments of public lands. US Geological Survey Open-File Reptor 94–203
Prohiæ E, Kniewald G (1987) Heavy metal distribution in recent sediments of the Krka river estuary—an example of sequential extraction analysis. Mar Chem 22:279–297
Rampe JJ, Runnells DD (1989) Contamination of water and sediment in a desert stream by metals from an abandoned gold mine and mill, Eureka District.. Appl Geochem 4:445–454
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer, Berlin Heidelberg New York
Shuman LM (1986) Effect of liming on the distribution of manganese, copper, iron, and zinc among soil fractions. Soil Sci Soc Am J 50:1236–1240
Smith KS, Ranville JF, Plumlee GS, Macalady DL (1998) Predictive double-layer modeling of metal sorption in mine-drainage systems. In: Jenne EA (eds) Adsorption of Metals by Geomedia. Academic, San Diego, pp 522–547
So CS, Yun ST, Kwon SH (1999) Gold–silver mineralization of the Jungheung and Okdong mines, Kwangyang area, Korea: mineralogical and geochemical change in a cooling hydrothermal system. Neues Jahrb Miner Abh 174:223–248
Sutherland RA (2002) Comparison between non-residual Al, Co, Cu, Fe, Mn, Ni, Pb and Zn released by a three-step sequential extraction procedure and a dilute hydrochloric acid leach for soil and road deposited sediment. Appl Geochem 17:353–365
Tessier A, Campbell PGC (1987) Partitioning of trace metals in sediments: relationships with bioavailability. Hydrobiologia 149:43–52
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–850
Tessier A, Campbell PGC, Bisson M (1980) Trace metal speciation in the Yamaska and St. Francois Rivers (Quebec). Can J Earth Sci 17:90–105
Tessier A, Rapin F, Carignan R (1985) Trace metals in oxic lake sediments: possible adsorption onto iron oxyhydroxides. Geochim Cosmochim Acta 49:183–194
Tessier A, Carignan R, Dubreuil B, Rapin F (1989) Partitioning of zinc between the water column and the oxic sediments in lakes. Geochim Cosmochim Acta 53:1511–1522
Trefry JH, Metz S (1984) Selective leaching of trace metals from sediments as a function of pH. Anal Chem 56:745–749
Yun ST, Choi BY, Lee PK (2000) Distribution of heavy metals (Cr, Cu, Zn, Pb, Cd, As) in roadside sediments, Seoul metropolitan city, Korea. Environ Technol 21:989–1000
Yun ST, Jung HB, So CS (2001) Transport, fate and speciation of heavy metals (Pb, Zn, Cu, Cd) in mine drainage: geochemical modeling and anodic stripping voltammetric analysis. Environ Technol 22:749–770
Zhang J, Huang WW, Wang JH (1994) Trace metal chemistry of the Huanghe (Yellow River), China—Examination of the data from in situ measurements and laboratory approach. Chem Geol 114:83–94
Acknowledgements
This work was financially supported by the Environmental Geosphere Research Lab (EGRL), Korea University, as an Advanced Basic Science Research Lab (ABRL) sponsored by Korea Science and Engineering Foundation (KOSEF). Field survey was partly supported by Korea Institute of Geoscience and Mineral Resources (KIGAM). Many comments provided by anonymous journal reviewers were very helpful to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, HB., Yun, ST., Mayer, B. et al. Transport and sediment–water partitioning of trace metals in acid mine drainage: an example from the abandoned Kwangyang Au–Ag mine area, South Korea. Environ Geol 48, 437–449 (2005). https://doi.org/10.1007/s00254-005-1257-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-005-1257-7