Abstract
Introduction
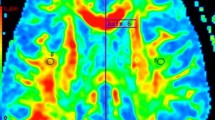
Clinical trials of secondary progressive multiple sclerosis (SPMS) is lacking reliable biomarkers or outcome measures that reflect tissue injury incurred within a 1- to 2-year observation period. Diffusion tensor imaging (DTI) is sensitive in detecting acute brain tissue damage. We monitored SPMS patients over 12 months for diffusion changes within the corpus callosum (CC).
Methods
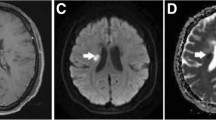
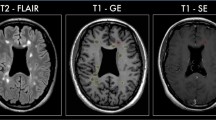
Bimonthly MRI examinations over a 1-year period were performed on 11 SPMS patients. The protocol included postcontrast T1-weighted images and DTI. Based on the appearance of T1 enhancing lesion(s) during the study period, the patients were divided into enhancing (five patients) and nonenhancing (six patients) groups. Fractional anisotropy (FA) and mean diffusivity (MD) of the genu, body, and splenium of the CC were measured and temporal changes in mean FA and MD were evaluated for each group as well as between groups. Immunology data from peripheral blood mononuclear cells were also collected on a monthly basis.
Results
The enhancing group showed significant, progressive decrease in FA in body (p = 0.012) and splenium (p = 0.033) of CC, and significantly higher lymphotoxin-β levels. No significant FA changes were seen in the nonenhancing group. Moreover, the FA decline in the enhancing group deviated significantly from the nonenhancing group, which remained essentially stable. Although MD increased slightly in both groups, there was no significant difference between the two groups.
Conclusion
Based on the MR and immunology findings, the results of our study suggest that DTI undergo more rapid and longitudinal changes in SPMS patients with inflammatory activity.




Similar content being viewed by others
References
Miller DH, Grossman RI, Reingold SC et al (1998) The role of magnetic resonance techniques in understanding and managing multiple sclerosis. Brain 121:3–24
Katz D, Taubenberger JK, Cannella B et al (1993) Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol 34:661–669
Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis: results of an international survey—National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 46:907–911
Grossman RI, Braffman BH, Brorson JR et al (1988) Multiple sclerosis: serial study of gadolinium-enhanced MR imaging. Radiology 169:117–122
Fillippi M, Rossi P, Colombo B et al (1997) Serial contrast-enhanced MR in patients with multiple sclerosis and varying levels of disability. AJNR 18:1549–1556
Guo AC, Macfall JR, Provenzale JM et al (2002) Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology 222:729–736
Werring DJ, Brassat D, Droogan AG et al (2000) The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain 123:1667–1676
Bammer R, Augustin M, Siegrid SF et al (2000) Magnetic resonance diffusion tensor imaging for characterization diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med 44:583–591
Ge Y, Law M, Johnson G et al (2004) Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 20:1–7
Ranjeva JP, Pelletier J, Gouny SC et al (2003) MRI/MRS of corpus callosum in patients with clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler 9:554–565
Hanson MK, Gupta KR, Santos MR (2005) Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J Magn Reson Imaging 21:735–743
Oh J, Henry RG, Genain C (2004) Mechanisms of normal appearing corpus callosum injury related to pericallosal T1 lesions in multiple sclerosis using directional diffusion tensor and 1 H MRS imaging. J Neurol Neurosurg Psychiatry 75:1281–1286
Lou X, Jiang WJ, Ma L et al (2009) Lower fractional anisotropy at the anterior body of the normal-appearing corpus callosum in multiple sclerosis versus symptomatic carotid occlusion. Neuroradiology 51:557–561
Polman CH, Reingold SC, Edan G et al (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Miller DH, Barkhof F, Berry I et al (1991) Magnetic resonance imaging in the monitoring treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry 54:683–688
Andersson JH, Square S (2002) A model-based method for retrospective correction of geometric distortions in diffusion-weighted EPI. Neuroimage 16:177–199
Ni H, Kavcic V, Zhu T et al (2006) Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR 27:1776–1781
Gupta RK, Saksena S, Hanson KM et al (2006) Focal Wallerian degeneration of the corpus callosum in large middle cerebral artery stroke: serial diffusion tensor imaging. J Magn Reson Imaging 24:549–555
Agosta F, Absinta M, Sormani MP et al (2007) In vivo assessment of cervical cord damage in MS patients: a longitudinal diffusion tensor MRI study. Brain 130:2211–2219
Filippi M, Yousry T, Campi A et al (1996) Comparison of triple dose versus standard dose gadolinium-DTPA for detection of MRI enhancing lesions in patients with MS. Neurology 46:379–384
Thompson AJ, Kermode AG, MacManus DG et al (1990) Patterns of disease activity in MS: clinical and MRI study. Br Med J 300:631–634
Matusevicius D, Navikas V, Söderström M et al (1996) Multiple sclerosis: the proinflammatory cytokines lymphotoxin-α and tumour necrosis factor-α are upregulated in cerebrospinal fluid mononuclear cells. J Neuroimmunol 66:115–123
Aboitiz F, Scheibel AB, Fisher RS et al (1992) Fiber composition of the human corpus callosum. Brain Res 598:143–153
Schmierer K, Altmann DR, Kassim N et al (2004) Progressive change in primary progressive multiple sclerosis normal-appearing white matter: a serial diffusion magnetic resonance imaging study. Mult Scler 10:182–187
Cassol E, Ibarrola D, Ranjeva JP et al (2004) Diffusion tensor imaging in multiple sclerosis: a tool for monitoring changes in normal-appearing white matter. Mult Scler 10:188–196
Tubridy N, Coles AJ, Molyneux P et al (1998) Secondary progressive MS: the relationship between short-term MRI activity and clinical features. Brain 121:225–231
Barkhof F, Held U, Simon JH et al (2005) Predicting gadolinium enhancement status in MS patients eligible for randomized clinical trials. Neurology 65:1447–1454
Evangelou N, Esiri MM, Smith S et al (2000) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47:391–395
Evangelou N, Konz D, Esiri MM et al (2000) Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 123:1845–1849
Bjartmar C, Wuiek JR, Trapp BD (2003) Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci 206:165–171
Uysal E, Erturk SM, Yildirim H et al (2007) Sensitivity of immediate and delayed gadolinium-enhanced MRI after injection of 0.5 M and 1.0 M gadolinium chelates for detecting multiple sclerosis lesions. AJR Am J Roentgenol 188:697–702
Acknowledgments
We are very grateful to Bill Badger for his help with data collection. This work was supported by an Autoimmunity Center of Excellence grant (1 U 19 AI056390-01; Project 2) from the NIAID, NIH (to B.M. Segal) and a Dana Foundation research grant (to B.M. Segal)
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tian, W., Zhu, T., Zhong, J. et al. Progressive decline in fractional anisotropy on serial DTI examinations of the corpus callosum: a putative marker of disease activity and progression in SPMS. Neuroradiology 54, 287–297 (2012). https://doi.org/10.1007/s00234-011-0885-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-011-0885-8