Abstract
Objective
The aim of this study was to investigate the pharmacokinetics of loratadine and its active metabolite desloratadine after single-dose administration of loratadine as a conventional tablet, orally disintegrating tablet (smelt tablet) and a chewing gum formulation with and without the collection of saliva.
Methods
Twelve healthy male volunteers participated in a four-period cross-over trial evaluating the effect of dosage forms on the pharmacokinetics of a single dose of loratadine. Loratadine was administered as two 10-mg conventional tablet, two 10-mg smelt tablet, a 30-mg portion of medicated chewing gum without collection of saliva and a 30-mg portion of medicated chewing gum with collection of saliva. Blood samples were taken at predefined sampling points 0–24 h after medication, and the plasma concentrations of loratadine and desloratadine were determined by high-performance liquid chromatography. Each study period was separated by a wash-out period of at least 7 days.
Results
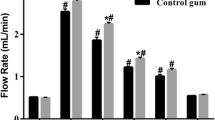
The mean dose-corrected area under the plasma concentration-time curve extrapolated to infinity AUC(0−∞) for the chewing gum formulation was statistically significantly increased compared to the tablet formulation (geometric mean ratio: 2.68; 95%CI: 1.75–4.09). Desloratadine pharmacokinetic parameters from the chewing gum formulation were not statistically significantly different from the conventional tablet. Neither loratadine nor desloratadine pharmacokinetics of the smelt tablet formulation were statistically significantly different from the conventional tablet formulation. Plasma concentrations of desloratadine following the administration of loratadine as chewing gum with saliva collection were very low.
Conclusion
Our study showed that formulation of loratadine as a medicated chewing gum results in an almost threefold increase in relative bioavailability. This is most likely due to a bypass of first-pass metabolism as this study suggests that approximately 40% of the absorbed loratadine was absorbed via the oral mucosa.



Similar content being viewed by others
References
Woodford DW and Lesko LJ (1981) Relative bioavailability of aspirin gum. J Pharm Sci 70(12):1341–1343
Jacobsen J, Christrup LL, Jensen NH (2004) Medicated chewing gum: pros and cons. Am J Drug Delivery 2(2):75–88
Bousquet E, Tirendi S, Bonina FP, Montenegro L, Bianchi A, Ciampini N (1992) Bioavailability of two formulations of acetylsalicylic acid gums. Pharmazie 47(8):607–609
Christrup LL, Bonde J, Eriksen H, Rasmussen SN, Rassing MR, Simonsen K (1988) Chewing gum as a drug delivery system III. Bioavailability of salicylamide administered in tablets and chewing gum. FARMACI, Sci Ed 16:6–14
Christrup LL, Bonde J, Rasmussen SN, Rassing MR (1990) Relative bioavailability of (+/−)-verapamil hydrochloride administered in tablets and chewing gum. Acta Pharm Nord 2(6):371–376
Benowitz NL, Jacob PI, Savanapridi C (1987) Determinants of nicotine intake while chewing nicotine polacrilex gum. Clin Pharmacol Ther 41(4):467–473
Hilbert J, Radwanski E, Weglein R, Luc V, Perentesis G, Symchowicz S, Zampaglione N (1987) Pharmacokinetics and dose proportionality of loratadine. J Clin Pharmacol 27(9):694–698
Yumibe N, Huie K, Chen KJ, Snow M, Clement RP, Cayen MN (1996) Identification of human liver cytochrome P450 enzymes that metabolize the nonsedating antihistamine loratadine. Formation of descarboethoxyloratadine by CYP3A4 and CYP2D6. Biochem Pharmacol 51(2):165–172
Katchen B, Cramer J, Chung M, Gural R, Hilbert J, Luc V, Mortizen V, Dsouza R, Symchowicz S, Zampaglione N (1985) Disposition of C-14-Sch 29851 in Humans. Ann Allergy 55(2):393
Pedersen RS, Damkier P, Brosen K (2005) Tramadol as a new probe for CYP2D6 phenotyping – a population study. Clin Pharmacol Ther 77(6):458–467
Shannon IL, Segreto VA (1968) Saliva specific gravity. Tech Rep SAM-TR 1–8
Dawes C (2006) Absorption of urea through the oral mucosa and estimation of the percentage of secreted whole saliva inadvertently swallowed during saliva collection. Arch Oral Biol 51(2):111–116
Zhang YF, Chen XY, Zhong DF, Dong YM (2003) Pharmacokinetics of loratadine and its active metabolite descarboethoxyloratadine in healthy Chinese subjects. Acta Pharmacol Sin 24(7):715–718
Lamba JK, Lin YS, Schuetz EG, Thummel KE (2002) Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev 54(10):1271–1294
Bradford LD (2002) CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 3(2):229–243
Yin OQ, Shi XJ, Tomlinson B, Chow MS (2005) Effect of cyp2d6*10 allele on the pharmacokinetics of loratadine in chinese subjects. Drug Metab Dispos 33(9):1283–1287
Acknowledgements
This study was supported by an unrestricted grant from Fertin Pharma A/S, Vejle, Denmark. The procedures in this study complied with the laws of Denmark, in which country they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Noehr-Jensen, L., Damkier, P., Bidstrup, T.B. et al. The relative bioavailability of loratadine administered as a chewing gum formulation in healthy volunteers. Eur J Clin Pharmacol 62, 437–445 (2006). https://doi.org/10.1007/s00228-006-0139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0139-6