Abstract
Purpose
This study was designed to optimize the latest generation venovenous (vv)-extracorporeal membrane oxygenation (ECMO)-circuit configuration and settings based on the evaluation of blood oxygenation and CO2 removal determinants in patients with severe acute respiratory distress syndrome (ARDS) on ultraprotective mechanical ventilation.
Methods
Blood gases and hemodynamic parameters were evaluated after changing one of three ECMO settings, namely, circuit blood flow, FiO2ECMO (fraction of inspired oxygen in circuit), or sweep gas flow ventilating the membrane, while leaving the other two parameters at their maximum setting.
Results
Ten mechanically ventilated ARDS patients (mean age 44 ± 16 years; 6 males; mean hemoglobin 8.0 ± 1.8 g/dL) on ECMO for a mean of 9.0 ± 3.8 days) receiving femoro–jugular vv-ECMO were evaluated. vv-ECMO blood flow and FiO2ECMO determined arterial oxygenation. Decreasing the ECMO flow from its baseline maximum value (5.8 ± 0.8 L/min) to 40 % less (2.4 ± 0.3 L/min) significantly decreased mean PaO2 (arterial oxygen tension; 88 ± 24 to 45 ± 9 mm Hg; p < 0.001) and SaO2 (oxygen saturation; 97 ± 2 to 82 ± 10 %; p < 0.001). When the ECMO flow/cardiac output was >60 %, SaO2 was always >90 %. Alternatively, the rate of sweep gas flow through the membrane lung determined blood decarboxylation, while PaCO2 (arterial carbon dioxide tension) was unaffected when the ECMO blood flow and FiO2ECMO were reduced to <2.5 L/min and 40 %, respectively. In three additional patients evaluated before and after red blood cell transfusion, O2 delivery increased after transfusion, allowing lower ECMO flows to reach adequate SaO2.
Conclusions
For severe ARDS patients receiving femoro–jugular vv-ECMO, blood flow was the main determinant of arterial oxygenation, while CO2 elimination depended on sweep gas flow through the oxygenator. An ECMO flow/cardiac output >60 % was constantly associated with adequate blood oxygenation and oxygen transport and delivery.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is a severe lung disease with a high mortality rate [1–4]. Patients acquiring its most severe forms, with severe hypoxemia, have the worst prognosis, and mortality can exceed 60 % [5]. In these situations, extracorporeal pulmonary assistance (oxygenation and CO2 removal from the blood), also known as extracorporeal membrane oxygenation (ECMO) [6, 7], is a therapeutic option that may minimize the trauma caused by mechanical ventilation (MV) and allow the lungs to rest while recovering. However, most trials evaluating ECMO for this indication over the past few decades [8, 9] have demonstrated no benefit because of the prolonged interval between ARDS onset and assistance initiation, the poor oxygenation and CO2-removal capacities of the devices used, and the high rate of apparatus-linked complications (significant bleeding resulting from intense anticoagulation required to overcome the poor biocompatibility of the circuits).
In recent years, major progress has been made in the manufacture of ECMO circuits, and they have become more biocompatible, with a higher performance and increased durability. The encouraging results of the CESAR trial [10] and the good outcomes of patients who received the latest generation of ECMO as rescue therapy during the recent A(H1N1) influenza pandemic [11–13] have reignited interest in ECMO for severe ARDS.
This study was designed to optimize the latest generation of venovenous (vv)-ECMO circuit configuration and settings based on an evaluation of blood oxygenation and CO2 removal determinants in patients with severe ARDS who were on ultraprotective MV with a restrictive transfusion policy.
Patients and methods
This study was conducted between October 2009 and April 2010 in the medical–surgical Intensive Care Unit (ICU) of the Hôpital de la Pitié–Salpêtrière (Paris, France). The protocol was approved by the hospital’s Institutional Review Board. Informed consent was obtained from all patients or their surrogates.
Patients
Despite optimized protective MV and other adjuvant treatment (i.e., prone positioning, inhaled nitric oxide, recruitment maneuvers), ten ARDS patients received vv-ECMO due to an arterial oxygen tension/fraction of inspired oxygen (PaO2/FiO2) of <80 mmHg and/or severe respiratory acidosis. Additional details are provided in the Electronic Supplementary Material (ESM).
Study protocol
To assess the impact of ECMO settings on blood oxygenation and decarboxylation, as well as hemodynamic parameters, we conducted this study in three stages. First, ECMO flow was gradually decreased from 100 to 80 to 60, and finally to 40 % of its maximum value, while the FiO2ECMO and the fresh gas flow rate were kept at 100 % and 10 L/min, respectively. After a 10-min stabilization period with the ECMO flow returned to its baseline value, FiO2ECMO was gradually reduced from 100 to 90, 80, 70, 60, 50, and finally to 40 %, while the fresh gas flow rate and ECMO flow were maintained at their maximum values. Lastly, after another 10-min stabilization period with the FiO2ECMO returned to 100 %, sweep gas flow was gradually lowered to 10, 8, 6, 4, and finally to 2 L/min, while the ECMO flow and FiO2ECMO were left at their maximum values. During the study, ventilator settings and sedation were unchanged. If the peripheral oxygen saturation (SpO2) dropped below 80 %, the trial was stopped and ECMO flow, FiO2ECMO, or sweep gas flow was returned to its baseline value. Additional details on data acquisition are provided in the ESM.
Impact of red blood cell transfusion on O2 content and delivery
The ECMO-flow reduction trial was performed as described above on three additional patients before and after they had received red blood cell transfusion. The time between the two trials was <24 h. At each step of the trial, blood samples for gas analysis were drawn from the right atrium, oxygenator inlet and outlet, and pulmonary and peripheral arteries, and cardiac output was measured with Doppler echocardiography. CaO2 (arterial O2 concentration), DO2 (oxygen delivery), and membrane oxygenator O2 transfer were calculated.
Statistical analyses
Continuous variables were compared with Student’s t test, the Mann–Whitney U test. or analysis of variance (ANOVA), as appropriate. Categorical variables were compared using the chi-square test. Changes in blood gases or hemodynamic parameters when the ECMO settings were modified were compared using repeated-measures ANOVA. Correlations between continuous variables were assessed with Spearman’s correlation coefficient. Significance was defined as p < 0.05. Statistical analyses were computed with StatView ver. 5.0 software (SAS Institute, Cary, NC).
Results
Study population
Ten patients (mean ± standard deviation; age 44 ± 16 years; 6 males) whose clinical characteristics and ARDS causes are summarized in Table 1 were evaluated. At the time of the study, the mean blood hemoglobin concentration of these ten patients was 8.0 ± 1.8 g/dL, and they had been on MV and ECMO for 9.0 ± 3.8 and 5.1 ± 3.8 days, respectively. The mean Doppler-measured cardiac index was 3.3 ± 0.4 L/min. The ECMO system and ventilator settings during the study are given in Table 2. Six (60 %) patients survived to discharge.
ECMO flow reduction trial
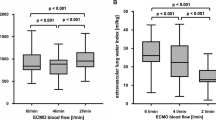
In the ECMO flow reduction trial, the ECMO flow was lowered from its baseline maximum value (5.8 ± 0.8 L/min) to 40 % of the maximum (2.4 ± 0.3 L/min) (Fig. 1). This resulted in a significant decline in mean oxygen pressure [PO2; 88 ± 24 (maximum ECMO) to 45 ± 9 mm Hg (40 % ECMO); p < 0.001] and oxygen saturation [SO2; 97 ± 2 (maximum ECMO) to 82 ± 10 % (40 % ECMO); p < 0.001]. Mean CaO2 decreased from 10.8 ± 2.2 mL O2/dL at 100 % ECMO flow to 8.5 ± 2.0 mL O2/dL at 40 % EMCO flow (p < 0.001).
Whisker plots illustrating the impact of extracorporeal membrane oxygenation (ECMO) flow reduction on oxygen pressure (PO 2 ), oxygen saturation (SO 2 ) and carbon dioxide pressure (PCO 2 ) in the right atrium, pulmonary artery, and peripheral artery. The impact was determined during the last step (i.e., 40 % of ECMO maximum flow) for seven patients. Horizontal line inside box Median, upper and lower box limits 25–75 percentiles, respectively, T-bars (whiskers) 10–90 percentiles
A strong linear correlation existed between the ECMO flow/cardiac output ratio and SaO2 (r 2 = 0.69, p < 0.0001) and PaO2 (r 2 = 0.80, p < 0.0001). The mean ECMO flow/cardiac output ratios were 76.5 ± 17.8 versus 48.3 ± 9.9 % for patients with SaO2 ≥90 % or <90 % (p < 0.001), respectively, and 84.2 ± 13.2 versus 54.2 ± 14.6 % for patients with PaO2 ≥60 mmHg or <60 mmHg, respectively, (p < 0.001). All patients whose ECMO flow/cardiac output exceeded 60 % had SaO2 >90 % and/or PaO2 >60 mmHg (Fig. 2 and ESM 2). Alternatively, decreasing the ECMO flow had no significant effect on arterial PCO2 (Fig. 1).
FiO2ECMO reduction trial
In the FiO2ECMO reduction trial, the ECMO flow (5.8 ± 0.8 L/min) and sweep gas flow (10 L/min) were maintained at their respective maximum and the FiO2ECMO was decreased. This resulted in a significant reduction of the PO2 and SO2 (p < 0.001) (Fig. ESM 2). Strong linear correlations were found between FiO2ECMO and SaO2 (r 2 = 0.68, p < 0.0001) and PaO2 (r 2 = 0.79, p < 0.0001). However, reducing the FiO2ECMO had no impact on arterial PCO2.
ECMO sweep gas flow reduction trial
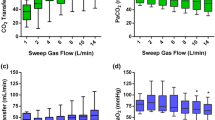
In the ECMO sweep gas flow reduction trial, decreases in the sweep gas flow rate through the membrane oxygenator had no significant impact on arterial PO2, but they were associated with significantly increased arterial PaCO2 (p < 0.001) and systolic pulmonary arterial pressure (p < 0.001) (Fig. 3). Right atrium and pulmonary artery occlusion pressures remained unchanged at each gas flow level (data not shown).
Whisker plots illustrating the impact of ECMO sweep gas flow reduction on arterial oxygen pressure (PaO 2 ), arterial carbon dioxide pressure (PaCO 2 ), and systolic pulmonary arterial pressure (sPAP). Decreasing the sweep gas flow through the membrane oxygenator had no significant impact on PaO2, but was associated with significantly increased PaCO2 (p < 0.001) and sPAP (p < 0.001). Horizontal line inside the box Median, upper and lower box limits 25–75 percentiles, respectively, T-bars (whiskers) 10–90 percentiles
Impact of red blood cell transfusion on O2 content and delivery
Hemoglobin levels for the three patients studied before and after a red blood cell transfusion were 5.1 and 8.1, 8.6 and 11.2, and 7.5 and 10.5 g/dL, respectively. The cardiac index remained stable when the PaO2, SaO2, and CaO2 decreased with reduced ECMO flow (Fig. ESM 3). The CaO2 and DO2 were higher after the transfusion (Table 3). Interestingly, while before the transfusion patients tolerated a reduction in the ECMO blood flow to only 60 % of the baseline value, it was possible after transfusion to decrease the ECMO flow to 40 % of the maximum value in two of the three patients while maintaining the SaO2 in the safe zone (>80 %). Membrane oxygenator O2 delivery was 104–149 mL/min (Table 3). Blood lactate remained within the normal range during these experiments.
Discussion
The results of this pilot physiological in vivo study demonstrate that, for patients who received vv-ECMO for refractory hypoxemia and whose native lung gas-exchange function was almost completely abolished, the factors determining arterial oxygenation were vv-ECMO blood flow and FiO2ECMO. Specifically, using the femoro–jugular ECMO setting, achieving a vv-ECMO flow of >60 % of systemic blood flow was consistently associated with an arterial blood saturation of >90 % and/or PaO2 of >60 mmHg. Alternatively, sweep gas flow rate ventilating the membrane lung determined blood decarboxylation, while arterial PCO2 was unaffected when the ECMO blood flow and FiO2ECMO were reduced to <2.5 L/min and 40 %, respectively. Lastly, high flows obtained through the ECMO circuit permitted adequate oxygen delivery (DO2) in our anemic patients. Increasing the transfusion threshold to 10 g/dL allowed a higher DO2 at a lower ECMO flow.
The main determinants of DO2 to peripheral tissues, which is critical for the preservation of organ function, are hemoglobin concentration, SaO2, and cardiac output [14]. When DO2 falls below a critical threshold, oxygen consumption becomes dependent on DO2 and the lactate concentration may increase, reflecting activation of anaerobic metabolism. To prevent tissue hypoxia in mechanically ventilated ARDS patients, an SaO2 ≥88 % using high positive end-expiratory pressure (PEEP) and high FiO2 are recommended [1, 15]. However, when refractory hypoxemia does develop, recourse to vv-ECMO is a reasonable therapeutic option [10, 12, 13, 16, 17]. In that context, blood oxygenation may become completely dependent on membrane-oxygenator oxygen-transfer capability, as was the case for the patients included in our study. Factors determining oxygenator oxygen transfer in this setting are blood-oxygen saturation in the ECMO drainage cannula, hemoglobin concentration, blood flow in the ECMO circuit, and intrinsic membrane-oxygenator properties, which depend on exchange-membrane surface and O2 diffusibility through hollow microfibers. When the blood flow through the ECMO circuit is >6 L/min, O2 transfer through the Quadrox® oxygenator (Quadrox, Henderson, NE) is theoretically >400 mL of O2/min, while oxygen saturation in the ECMO drainage canula is 70 % and hemoglobin concentration is 15 g/dL [18].
However, since both drainage and return cannulae are positioned within the venous system in vv-ECMO, blood recirculates into the oxygenator, i.e., some of the returned blood is once again drained into the circuit instead of passing through the right heart, thus markedly reducing O2 transfer efficiency [19]. To minimize blood recirculation, the circuit can be configured in several ways [16, 17]. In the bi-femoral configuration, the drainage cannula is positioned in the inferior vena cava and a femoral return cannula is advanced to the right atrium. However, 50 % of the patients who received bi-femoral vv-ECMO for A(H1N1)-induced ARDS in the ANZICS ICUs also needed a second (jugular) drainage cannula because of insufficient blood drainage [13].
Alternatively, a single bicaval dual-lumen cannula (Avalon Elite®; Avalon Laboratories, Los Angeles, CA) can be inserted via the right jugular vein and positioned to allow drainage from the inferior and superior vena cavae; oxygenated blood then returns via a second lumen to the right atrium [20]. This configuration minimizes blood recirculation, but insertion of the jugular catheter requires an experienced and skilled operator, as well as recourse to fluoroscopy or trans-esophageal echocardiography guidance for correct positioning. Because >50 % of our vv-ECMO patients receive emergency ECMO support at primary-care hospitals by our institution’s Mobile Circulatory/Respiratory Assistance Unit, we chose the femoro–jugular set-up for vv-ECMO, which is the most simple and rapid configuration. To minimize blood recirculation, it is important to ascertain that the tip of the return cannula is positioned away from that of the inflow cannula. Indeed, the mean distance between the two cannulae was 13 cm in our patients.
We chose the femoro–jugular configuration because it has also been shown that, compared to the jugulo–femoral set, it provides higher maximum ECMO flow and higher pulmonary artery mixed venous oxygen saturation and requires comparatively less flow to maintain an equivalent mixed venous oxygen saturation [21].
In terms of improving blood oxygen transfer in the oxygenator and increasing oxygen transport to peripheral organs, our findings demonstrate that in addition to the ECMO cannula configuration, ECMO flow through its circuitry is the major determinant of blood oxygenation. An ECMO flow of >60 % of systemic blood flow obtained adequate peripheral oxygenation. Thus, depending on patient size, cardiac output, oxygen consumption, and lung shunt, a circuit blood flow of 4–7 L/min will typically be required to achieve arterial oxygen saturations of >88–90 %, while maintaining safe lung ventilation. Therefore, large-sized (24–30 Fr) and multi-holed drainage cannula should be preferred to obtain high flows with reasonable negative pressure in that cannula. Indeed, when small cannulae are used with high flows, the suction created by the centrifugal pump can cause excessive depression and cavitation in the inflow line, resulting in massive intravascular hemolysis [16, 17].
The other important parameter that can be manipulated to enhance tissue DO2 and maximize oxygen transfer through the membrane oxygenator is the blood hemoglobin concentration [14]. Guidelines from the Extracorporeal Life Support Organization (ELSO) and the CESAR trial recommend maintaining a normal hematocrit (40–45 %) and hemoglobin concentrations at 14 g/dL, respectively, for patients on ECMO support [10, 22]. However, critically ill patients, and especially those already suffering from diffuse alveolar damage, may be at high risk of transfusion-related acute lung injury [23–25]. The transfusion of blood products increases volume, which might also complicate the ARDS course, as a slower improvement in lung function and a longer MV duration were reported when a liberal fluid-management strategy was applied to patients with acute lung injury [26].Therefore, at our hospital, we have been applying a restrictive transfusion strategy with the red blood cell transfusion threshold set at 7–8 g/dL in most patients on EMCO. Herein, despite a mean hemoglobin concentration and DO2 of 7–8 g/dL and 300–350 mL/min/m2, respectively, each patient in our study had adequate SaO2, and no sign of an oxygen uptake (VO2)/DO2 mismatch was observed. Nevertheless, in patients for whom SaO2 and DO2 remain low despite maximal ECMO flow or when high ECMO flows induce marked hemolysis, increasing the transfusion threshold to 10 g/dL might re-establish a sufficient oxygen reserve and adequate DO2 and allow lower ECMO flows.
CO2 transfer through the membrane lung also depends on ECMO flow, with the maximum transfer being >300 mL/min when the ECMO flow is >6 L/min with the Quadrox® oxygenator. However, since CO2 diffuses 20-fold faster than O2, large amounts of CO2 can be exchanged through the membrane lung even when flow through the circuit is low. This capacity was confirmed by our results showing that PaCO2 remained unchanged when ECMO blood flow was lowered to <2.5 L/min. Indeed, this property is the basis for developing low-flow extracorporeal CO2-removal devices, whose CO2 removal rate is 30–70 mL/min at blood flows of only 300–450 mL/min [27, 28]. Alternatively, sweep gas flow across the oxygenator is the main determinant of CO2 removal by ECMO. We observed a PaCO2 increase from 33 to 50 mmHg when the sweep gas flow was reduced from 10 to 2 L/min, which was paralleled by a significantly higher pulmonary artery pressure. While we observed no PaO2 change when sweep gas flow was decreased, a recent experimental study involving six patients treated with vv-ECMO and neurally adjusted ventilatory assist during the recovery phase of ARDS [29] reported that a 50 % reduction of the maximal sweep gas flow for 30 min resulted in a significant increase in the minute ventilation and PaO2 through the recruitment of previously non-ventilated areas. Indeed, Kolobow et al. [30] had shown in the 1970s that minute ventilation is directly coupled to extracorporeal CO2 elimination, and Karagiannidis et al. [29] confirmed that the main factor determining the upregulation of ventilation was not oxygenation, but the respiratory drive to achieve CO2 homeostasis and pH. The differences in PaO2 variation between that latter study and our findings can be explained by our patients having been evaluated during the early acute phase of ARDS, when deeply sedated; minute ventilation could therefore not be improved.
Some limitations of our study must be noted. First, all of our patients were hemodynamically stable with no catecholamine infusion during the study. Patients with ARDS caused by severe sepsis or septic shock might have higher cardiac output and impaired peripheral oxygen extraction. In that situation, even ECMO flows of up to 6 L/min might not achieve adequate blood oxygenation and DO2, particularly if the pulmonary gas-exchange capacity is severely impaired and ultraprotective (low volume, low pressure) MV is applied. In that context, every effort to decrease VO2 should be made (i.e., fever control, muscle paralysis), and the transfusion threshold should possibly be increased to >10 g/dL. Second, the use of a single double-lumen cannula was associated with less blood recirculation and a greater efficiency of the membrane oxygenator. Using this type of cannula might enable lower ECMO blood flow to reach similar oxygenation objectives. Third, when this study was conducted, we were using drainage cannulae with a smaller diameter(mean 23 Fr) compared to those used at the present time. We now use a 29-Fr multi-perforated femoro-atrial drainage cannula, which allows better blood drainage, enables a lower suction pressure, and is associated with less blood trauma. Under these conditions, reaching an ECMO blood flow of 6.5–7 L/min is possible with the drainage pressure remaining above −100 mmHg. Lastly, hemodynamic and blood-gas measurements in deeply sedated patients were obtained after a 10-min stabilization period. Therefore, we cannot exclude the possibility that signs of tissue hypoxia might have been observed if a longer stabilization period had been used and patients had received less sedation.
In conclusion, we demonstrated that in our patients with ARDS, ECMO flow was the main determinant of arterial oxygenation supported by vv-ECMO, while CO2 elimination mainly relied on sweep gas flow through the oxygenator. The femoro-jugular ECMO configuration we used permits easy and rapid vascular access that is well adapted to the emergency and patient-retrieval setting, and high ECMO flows when large-diameter drainage cannulae are used. In the context of our study, with hemoglobin maintained at around 7–8 g/dL for preventing transfusion-associated lung injury and worsening of lung damage, an ECMO flow/cardiac output ratio of >60 % was constantly associated with adequate blood oxygenation and oxygen transport and delivery. Increasing the transfusion threshold to 10 g/dL might re-establish adequate DO2 in patients for whom the SaO2 and DO2 remain low despite maximal ECMO flow.
References
Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L (2008) Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:646–655
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:637–645
Rubenfeld GD, Herridge MS (2007) Epidemiology and outcomes of acute lung injury. Chest 131:554–562
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693
Sud S, Friedrich JO, Taccone P, Polli F, Adhikari NK, Latini R, Pesenti A, Guerin C, Mancebo J, Curley MA, Fernandez R, Chan MC, Beuret P, Voggenreiter G, Sud M, Tognoni G, Gattinoni L (2010) Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 36:585–599
Schuerer DJ, Kolovos NS, Boyd KV, Coopersmith CM (2008) Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest 134:179–184
Chalwin RP, Moran JL, Graham PL (2008) The role of extracorporeal membrane oxygenation for treatment of the adult respiratory distress syndrome: review and quantitative analysis. Anaesth Intensive Care 36:152–161
Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, Dean NC, Thomas F, East TD, Pace NL et al (1994) Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149:295–305
Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC 2nd, Thomas AN, Proctor HJ, Drinker PA, Pratt PC, Bagniewski A, Miller RG Jr (1979) Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 242:2193–2196
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363
Patroniti N, Zangrillo A, Pappalardo F, Peris A, Cianchi G, Braschi A, Iotti GA, Arcadipane A, Panarello G, Ranieri VM, Terragni P, Antonelli M, Gattinoni L, Oleari F, Pesenti A (2011) The Italian ECMO network experience during the 2009 influenza A (H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 37:1447–1457
Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, Sadique MZ, Sekhon JS, McAuley DF, Firmin RK, Harvey C, Cordingley JJ, Price S, Vuylsteke A, Jenkins DP, Noble DW, Bloomfield R, Walsh TS, Perkins GD, Menon D, Taylor BL, Rowan KM (2011) Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 Influenza A(H1N1). JAMA 306:1659–1668
Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettila V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M (2009) Extracorporeal membrane oxygenation for 2009 Influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
Vincent JL, De Backer D (2004) Oxygen transport—the oxygen delivery controversy. Intensive Care Med 30:1990–1996
[No authors listed] (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
Aissaoui N, Luyt CE, Leprince P, Trouillet JL, Leger P, Pavie A, Diebold B, Chastre J, Combes A (2011) Predictors of successful extracorporeal membrane oxygenation (ECMO) weaning after assistance for refractory cardiogenic shock. Intensive Care Med 37:1738–1745
MacLaren G, Combes A, Bartlett RH (2012) Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 38:210–220
Jegger D, Tevaearai HT, Mallabiabarrena I, Horisberger J, Seigneul I, von Segesser LK (2007) Comparing oxygen transfer performance between three membrane oxygenators: effect of temperature changes during cardiopulmonary bypass. Artif Organs 31:290–300
Walker JL, Gelfond J, Zarzabal LA, Darling E (2009) Calculating mixed venous saturation during veno-venous extracorporeal membrane oxygenation. Perfusion 24:333–339
Bermudez CA, Rocha RV, Sappington PL, Toyoda Y, Murray HN, Boujoukos AJ (2010) Initial experience with single cannulation for venovenous extracorporeal oxygenation in adults. Ann Thorac Surg 90:991–995
Rich PB, Awad SS, Crotti S, Hirschl RB, Bartlett RH, Schreiner RJ (1998) A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg 116:628–632
Extracorporeal Life Support Organization (2009) ELSO guidelines. Available at: http://www.elso.med.umich.edu/Guidelines.html
Vlaar AP, Hofstra JJ, Determann RM, Veelo DP, Paulus F, Kulik W, Korevaar J, de Mol BA, Koopman MM, Porcelijn L, Binnekade JM, Vroom MB, Schultz MJ, Juffermans NP (2011) The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood 117:4218–4225
Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC (2005) Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 33:1191–1198
Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O’Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB (2007) Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med 176:886–891
Kacmarek RM, Wiedemann HP, Lavin PT, Wedel MK, Tutuncu AS, Slutsky AS (2006) Partial liquid ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 173:882–889
Terragni PP, Del Sorbo L, Mascia L, Urbino R, Martin EL, Birocco A, Faggiano C, Quintel M, Gattinoni L, Ranieri VM (2009) Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 111:826–835
Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC (2011) Respiratory dialysis: reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med 39:1382–1387
Karagiannidis C, Lubnow M, Philipp A, Riegger GA, Schmid C, Pfeifer M, Mueller T (2010) Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med 36(12):2038–2044
Kolobow T, Gattinoni L, Tomlinson TA, Pierce JE (1977) Control of breathing using an extracorporeal membrane lung. Anesthesiology 46:138–141
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schmidt, M., Tachon, G., Devilliers, C. et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 39, 838–846 (2013). https://doi.org/10.1007/s00134-012-2785-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2785-8