Abstract
Actin-directed processes such as membrane ruffling and cell migration are regulated by specific signal transduction pathways that become activated by growth factor receptors. The same signaling pathways that lead to modifications in actin dynamics also activate cPLA2α. Moreover, arachidonic acid, the product of cPLA2α activity, is involved in regulation of actin dynamics. Therefore, it was investigated whether cPLA2α plays a role in actin dynamics, more specifically during growth factor-induced membrane ruffling and cell migration. Upon stimulation of ruffling and cell migration by growth factors, endogenous cPLA2α and its active phosphorylated form were shown to relocate at protrusions of the cell membrane involved in actin and membrane dynamics. Inhibition of cPLA2α activity with specific inhibitors blocked growth factor-induced membrane and actin dynamics, suggesting an important role for cPLA2α in these processes.
Similar content being viewed by others
Introduction
Actin plays an essential role in a wide variety of dynamic events in mammalian cells, amongst them cell motility, cytokinesis, and cell–cell and cell–matrix interactions [1–4]. Actin dynamics are determined by the activities of a large number of actin-binding proteins. Extracellular stimuli, like growth factors and extracellular matrix components, stimulate intracellular signal transduction cascades that activate actin-binding proteins resulting in changes in local actin dynamics. These changes result in morphological changes like the formation of membrane ruffles and leading edges. In addition to changed actin dynamics upon growth factor addition or cell spreading, many signal transduction components are bound to actin, indicating the mutual relationship [5–7].
An important molecule involved in the regulation of cell metabolism is the unsaturated fatty acid arachidonic acid, which serves as a precursor for leukotriene and prostaglandin synthesis. However, arachidonic acid release has also been related to the regulation of actin dynamics [8]. In HeLa cells, an increase in arachidonic acid production was shown after cell attachment. This results in both actin polymerization and actin bundling via two distinct pathways involving lipoxygenase and cyclooxygenase [9, 10]. These enzymes metabolize arachidonic acid, resulting in the release of lipid second messengers that activate actin-binding proteins and consequently cause a change in actin dynamics. Additional to its role in cell spreading and immobilization, arachidonic acid was also shown to stimulate cell motility via the production of prostaglandins by cyclooxygenase. Prostaglandins play a role in actin cytoskeletal regulation and migration in a variety of ways. For example, stimulation of cells by chemokines resulted in an increase of prostaglandins and consequently in an increase of actin polymerization [11]. Moreover, several forms of stimulated migration require arachidonic acid metabolites [12, 13]. In addition, dissolution of adhesion structures like podosomes is dependent on the production of prostaglandins [14]. The role of arachidonic acid-mediated signaling in the regulation of small GTPases that modulate actin remodeling has been illustrated in several studies. In Swiss 3T3 fibroblasts, various leukotrienes induce stress fiber formation via a Rho-dependent process [15]. In Rat2 fibroblasts, arachidonic acid induces membrane ruffling and this is prevented when a dominant-negative mutant of Rac1 (Rac1N17) is expressed, indicating that Rac1 is activated downstream of arachidonic acid-mediated signaling [16]. Since Rac was also shown to act upstream of arachidonic acid release [15], it is possible that arachidonic acid and Rac1 act in a positive feedback loop.
The release of arachidonic acid in cells results from cleavage of membrane phospholipids at the sn-2 position by phospholipases A2 (PLA2). The large family of PLA2 consists of several sub-families like the secretory PLA2s (sPLA2), the intracellular calcium independent PLA2s (iPLA2), and the group of cytosolic PLA2s (cPLA2) including cPLA2α [17]. Interestingly, cPLA2α is highly specific for phospholipids carrying arachidonic acid at the sn-2 position and is therefore considered as the rate-limiting enzyme in receptor-mediated arachidonic acid release. cPLA2α requires Ca2+ to translocate from the cytosol to the membrane through its CaLB (calcium-lipid binding) domain, although Ca2+ is not directly involved in catalysis [18]. The activation of cPLA2α results from a phosphorylation by MAP kinase (for review, see [18]). This MAP kinase on its turn is activated by the same signal transduction cascades (growth factors and extracellular matrix components) that lead to modifications in actin dynamics. Therefore, it is tempting to suggest that cPLA2α is one of the key enzymes involved in local actin dynamics that are involved in the formation of membrane ruffles, and in cell motility.
Therefore, we have studied the localization of cPLA2α in fibroblasts during stimulation with platelet-derived growth factor (PDGF). Stimulation with PDGF introduces circular dorsal ruffles and lamellipodia. Here, we show that endogenous cPLA2α and its active, phosphorylated form are localized at the cell membrane in response to PDGF stimulation. They localize specifically to areas at the cell membrane that are reshaped by actin during PDGF stimulation. Furthermore, endogenous cPLA2α and its active phosphorylated form are shown to localize at leading edges in primary human umbilical vein endothelial cells (HUVECs) and migrating mouse fibroblasts. Interestingly, inhibition of cPLA2α activity with specific inhibitors blocked PDGF-induced membrane and actin dynamics, suggesting a role for cPLA2α in these processes.
Materials and methods
Cell culture
Human umbilical vein endothelial cells were isolated according to Jaffe [19] and used from passage 1 to 4. HUVECs were cultured onto fibronectin-coated surfaces and grown in endothelial basal medium (EBM-2) supplemented with 2% fetal bovine serum (FBS) (Gibco, Paisley, UK), endothelial cell growth supplements (EGM-2) (Cambrex, NJ, USA), and 5 mM l-glutamine (Invitrogen). C3H10T1/2 mouse fibroblasts were cultured in HEPES (25 mM) buffered Dulbecco’s modified Eagle’s medium (DMEM) (Gibco) supplemented with 7.5% FBS and 5 mM l-glutamine. Cells were grown at 37°C (humidified atmosphere, 5% CO2).
HUVECs were seeded on glass coverslips at 10,000 cells/cm2, coated with fibronectin and allowed to grow for 2 or more days to obtain sub-confluent monolayers for localization studies and confluent monolayers for the scratch assays.
Fibroblasts were plated on glass coverslips at 12,000 cells/cm2 and allowed to grow for 24 h. For PDGF stimulation experiments, cells were subsequently growth factor-starved for 24 h at 37°C and stimulated with 20 ng/ml of PDGF-BB (Upstate, Hampshire, UK). Treatment with cPLA2α inhibitors Pyrrolidine-2 (2 μM) and Wyeth-1 (5 μM) was performed in serum-free medium. Both inhibitors were kind gifts from Prof. Dr. M. Gelb (University of Washington, Seattle, USA).
Immunofluorescent labeling
Cells were fixed in 3.2% formaldehyde for 15 min, washed twice with PBS, permeabilized for 5 min in PBS containing 0.2% Triton X-100, followed by two washes in PBS, and incubated for 10 min with 50 mM glycine in PBS. After washing twice with PBS containing 0.2% gelatin, cells were incubated overnight with goat anti-cPLA2 (Santa Cruz; sc-1724) or for 1 h with rabbit anti-phospho-cPLA2 (ser505) (Santa Cruz; sc-34391). Then, the cells were washed six times with PBS containing 0.2% gelatin and incubated for 1 h with tetramethylrhodamine-5-(and-6)-isothiocyanate (TRITC)-conjugated phalloidin (Sigma) combined with goat-anti-rabbit-Alexa488 or donkey-anti-goat-Alexa488 (Molecular Probes) and washed six times PBS containing 0.2% gelatin. In some samples, this was followed by a DAPI staining for 10 min. Finally, cells were mounted in Mowiol-DABCO.
Transfections
Transfections with GFP-cPLA2α were performed using Lipofectin (Invitrogen) according to the manufacturer’s instructions. This construct, kindly provided by Dr. C. Lenoir (Inserm Unit 538, Paris, France), was checked by sequencing.
Scratch assay
HUVECs were seeded on fibronectin-coated glass coverslips at 10,000 cells/cm2 (~10–20% confluency) and allowed to grow for 7 days with regular change of medium. Cells were gently scratched from the substrate using a pipette tip. The remaining cells were washed with culture medium and allowed to reoccupy the wounded area for various time intervals. Mouse C3H10T1/2 fibroblasts were seeded at 20,000 cells/cm2 and allowed to grow to confluency before performing the scratch assay as described above.
Acquisition of images
Samples were studied with a Leitz microscope (Orthoplan Flu 043944) equipped with Leitz oil objectives ×40 NA 1.3 and ×63 NA 1.4. Images were acquired using a Leica CCD camera (model DC350F; Leica Microsystems) using Leica Image Manager 50 software. Confocal pictures were obtained using a Zeiss CLSM (Pascal 510) equipped with Zeiss water objectives (×40 NA 1.3 and ×63 NA 1.4). Images were processed with Adobe Photoshop 8.0.
Results
cPLA2α is recruited to PDGF-induced dorsal membrane ruffles and lamellae in C3H10T1/2 fibroblasts
Mouse C3H10T1/2 fibroblasts provide an excellent model cell line to study actin dynamics. When these cells are serum-starved for 24 h, they are spread and exhibit a clear network of actin stress fibers as shown by staining of F-actin with phalloidin (Fig. 1b). Addition of PDGF-BB, (20 ng/ml for 15 min at 37°C), results in the partial disappearance of stress fibers, and the formation of large circular dorsal membrane ruffles, characterized by a ring of F-actin beneath the membrane (Fig. 1e). Successive optical sections obtained by confocal scanning light microscopy confirmed that these ruffles extend from the dorsal plasma membrane (Fig. 1e, h). As reported previously, ruffle formation is dependent upon actin polymerization and is highly transient [20]. Since actin polymerization is induced by growth factors [21] and is dependent on the production of arachidonic acid metabolites [8, 9], it is tempting to suggest that cPLA2α plays a role in actin dynamics, because cPLA2α is also activated by growth factors [22–24]. Therefore, the subcellular localization of endogenous cPLA2α was determined upon PDGF-induced changes in actin polymerization. Cells were labeled with an antibody specific for cPLA2α as characterized previously [25]. As shown in Fig. 1a, in serum-starved fibroblasts, cPLA2α is mainly detected in the perinuclear area and throughout the cytoplasm. Upon addition of PDGF-BB to the cells, cPLA2α is recruited to newly formed circular dorsal ruffles (Fig. 1d, g) as demonstrated by the colocalization of cPLA2α and PDGF-induced F-actin structures (Fig. 1f, i). This result indicates that upon growth factor stimulation, cPLA2α is rapidly and specifically recruited at areas of actin polymerization at the plasma membrane.
Relocalization of cPLA2α to dorsal membrane ruffles and to lamellae in C3H10T1/2 fibroblasts upon PDGF stimulation. Fibroblasts were serum deprived for 24 h and subsequently incubated in the presence (d–i, m–o) or absence (control: a–c, j–l) of 20 ng/ml PDGF-BB for 10 min (j–o) or 15 min (a–i) at 37°C. Cells were fixed and subjected to immunofluorescent staining (cPLA2α green, F-actin red) and samples were analyzed by confocal microscopy. In non-stimulated cells, cPLA2α is found in the perinuclear area and in the cytoplasm (a, j) and F-actin is organized in large stress fibers (b, k). Stimulation with PDGF-BB results in the partial disappearance of stress fibers (e,h,n), in the formation of circular dorsal ruffles (e,h,f,i, arrowheads) to which cPLA2α is recruited (d,g), and in the formation of lamellae (n,o, arrowheads) in which cPLA2α accumulates (m, arrowhead). Panels d–f and g–i are images taken from the same cell but d–f is a section at 0.88 μm through the cytoplasm, the nucleus, and the base of the circular ruffle whereas g–i represents a section at 3.08 μm through the top of the nucleus and the protrusion of the circular ruffle. Bar 10 μm
In addition to the formation of membrane ruffles, C3H10T1/2 cells also form lateral lamellae upon addition of PDGF (Fig. 1n). Whether the cells form lamellae or circular ruffles is determined by the PDGF concentration used. At concentrations >20 ng/ml, abundant circular ruffling takes place, whereas at concentrations in the range of 1–5 ng/ml, less circular dorsal ruffles occur and more lamellae are formed. The formation of lamellae is related to cell spreading and/or cell motility. As clearly shown in Fig. 1n, treatment of cells with 20 ng/ml PDGF for 10 min at 37°C results in the formation of lamellae characterized by a lateral band of newly formed F-actin (Fig. 1n) in which cPLA2α accumulates (Fig. 1m), while the perinuclear localization of cPLA2α, as observed in untreated cells (Fig. 1j), disappeared. Together these data clearly demonstrate that PDGF-induced actin remodeling is accompanied by a recruitment of cPLA2α to sites of new actin polymerization and membrane dynamics.
cPLA2α is localized at leading edges of migrating mouse fibroblasts
To determine whether the recruitment of cPLA2α at sites of newly formed F-actin represents a feature common to other cellular processes that depend on actin polymerization, the localization of cPLA2α was determined in the context of cell migration. We performed a commonly used wound-healing assay, also called scratch assay. In this assay, a confluent monolayer is gently scratched with a pipette tip to introduce a wound. Cells located at the border of the wound respond by polarizing towards the wound, emit protrusions, and start to migrate into the wounded area which is finally healed. Directly after scratching monolayers of C3H10T1/2, cPLA2α was mainly cytoplasmic and perinuclear in cells lining the wound (Fig. 2b). Seven hours after the scratch, cells located at the border of the wound displayed protrusions of the plasma membrane in which cPLA2α was detected (Fig. 2e, f). This membrane localization was restricted to the leading edge of the migrating cells. These edges are enriched in F-actin (Fig. 2d) and in ARP 3 indicating active actin remodeling (data not shown). At later time points, cPLA2α was detected at leading edges of cells that had migrated into the wound. In conclusion, these data show that in migrating cells, endogenous cPLA2α is recruited to the leading edges of migrating cells, i.e. at sites of active actin remodeling.
cPLA2α localizes at the leading edge in migrating mouse fibroblasts and HUVECs. Confluent monolayers of C3H10T1/2 fibroblasts were subjected to the scratch assay. Cells were fixed immediately after scratch (a–c) or 7 h after scratch (d–f) and subjected to immunofluorescent staining (cPLA2α red, F-actin green) and samples were analyzed by confocal microscopy. In confluent mouse fibroblasts, cPLA2α is found mainly cytoplasmic directly after scratch (b). After 7 h, cells located at the border of the wound have migrated in the wounded area and cPLA2α has translocated from the cytoplasm to the newly formed leading edges (e). Confluent monolayers of HUVEC were subjected to the scratch assay and processed for immunofluorescence immediately after scratch (g,h) or 22 h after scratch (j–l). In confluent HUVECs cPLA2α is located at the Golgi directly after scratch (h). At 22 h after scratch, in cells that have migrated into the wounded area, cPLA2α is more abundant in the cytoplasm and has translocated to the newly formed leading edges. Insert shows a leading edge where cPLA2α colocalizes with F-actin. Bar 10 μm
cPLA2α is localized at leading edges of migrating primary human endothelial cells
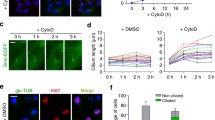
To determine whether the recruitment of cPLA2α at sites of actin polymerization as observed in fibroblasts is a conserved phenomenon, the relationship between cPLA2α and F-actin was studied in primary endothelial cells of human origin, i.e. HUVECs. It has been demonstrated that cPLA2α translocates to the Golgi complex in response to confluency in HUVECs [25, 26]. A scratch assay was performed on a confluent monolayer of HUVECs and the localization of cPLA2α and F-actin was examined. Immediately after scratch, cPLA2α was localized at the Golgi apparatus in all cells (lining the wound or not) (Fig. 2h). Four hours after the scratch, cells located at the border of the wound displayed protrusions of the plasma membrane in which cPLA2α was detected and where cPLA2α locally co-localized with F-actin (data not shown). Subsequently migrating cells reoccupy the wounded area. Twenty-two hours after scratch, migrating cells filled up the wounded area (Fig. 2j, k, l) and displayed cPLA2α staining at the cell membrane (Fig. 2k) and leading edge (Fig. 2k insert), where it locally colocalizes with F-actin. In contrast to confluent cells (Fig. 2h), in the wounded area, cPLA2α is localized primarily in the cytoplasm and also at the cell periphery (Fig. 2k). This latter localization may both be related to cell spreading and cell migration.
To determine whether the recruitment of cPLA2α to leading edges of migrating HUVECs was not solely occurring in the context of the scratch assay, the localization of cPLA2α was compared in non-confluent versus confluent cultures. In contrast to confluent cultures, in which HUVECs are growth-arrested by contact inhibition and are unable to migrate, cells in non-confluent cultures do proliferate and migrate. Both migration and cell proliferation are dependent on actin polymerization, and therefore non-confluent cells display numerous and extended membrane areas where actin polymerization takes place. In confluent HUVECs, cPLA2α is located at the Golgi and does not co-localize with F-actin (Fig. 3c). In contrast, in non-confluent cells that display a migratory phenotype, cPLA2α is present at the cell periphery in restricted membrane areas which correspond to sites of actin polymerization (Fig. 3b, d).
Subcellular localization of endogenous cPLA2α in non-confluent and confluent HUVEC. Non-confluent or confluent HUVECs were stained for cPLA2α (green), F-actin (red) and the nuclei were stained with DAPI (blue). In confluent HUVECs (a,c) cPLA2α is localized at the Golgi; in non-confluent cells (b,d) cPLA2α is localized both in the perinuclear area and at specific areas of the plasma membrane (arrowheads) corresponding to sites of actin polymerization
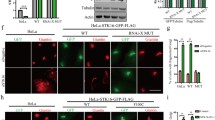
The goat anti-cPLA2α antibody used in our study is specific for cPLA2α (confirmed by western blotting of HUVEC and C3H10T1/2 lysates (data not shown and [26–28]). However, to further confirm the presence of cPLA2α at the cell membrane, we examined the subcellular localization of GFP-tagged human cPLA2α in transiently transfected HUVECs. In non-confluent cells, GFP-cPLA2α was localized at the cell membrane, in addition to its cytoplasmic and perinuclear localization (Fig. 4d, f). This confirms our data on the localization of endogenous cPLA2α at the cell membrane in leading edges. Additionally, GFP-cPLA2α was detected at the Golgi apparatus of confluent HUVECs (Fig. 4a), showing that GFP-cPLA2α exhibits the same translocation property as endogenous cPLA2α. Finally, GFP-cPLA2α was recognized by the goat anti-cPLA2 antibody (Fig. 4b, c).
Subcellular localization of GFP-cPLA2α in non-confluent and confluent HUVECs. Confluent (a–c) and non-confluent (d–f) HUVECs were transfected with the GFP-cPLA2α construct. Confluent cells were fixed and stained with the goat polyclonal anti-cPLA2α (green GFP-cPLA2α, red cPLA2α). a–c show that the GFP-cPLA2α signal (a) is recognized by the antibody against cPLA2α (b). Non-confluent HUVEC transfected with GFP-cPLA2α were stained with DAPI (blue) to visualize the nuclei. In these cells, GFP-cPLA2α was mainly located in the cytoplasm and in the perinuclear area and was also found at specific areas of the plasma membrane. Bar 10 μm
Phosphorylated cPLA2α is present in circular dorsal ruffles and leading edges
In view of our data demonstrating the recruitment of cPLA2α at sites of actin remodeling and the possible involvement of the arachidonic acid pathway in actin remodeling, it is tempting to suggest that the enzymatic activity of cPLA2α is important for this process. Since phosphorylation of cPLA2α at serine residue 505 by mitogen-activated protein kinases (MAPK) is involved in cPLA2α activation [29], we studied the localization of phosphorylated cPLA2α using an antibody specific for Ser505-phosphorylated cPLA2α. In cultures of serum-starved mouse fibroblasts, phospho-S505-cPLA2α was detected in the cytoplasm and in the nucleus of most cells (Fig. 5a). After stimulation with PDGF for 15 min, phospho-S505-cPLA2α was found in circular dorsal ruffles (Fig. 5d). Additionally, phospho-S505-cPLA2α was detected at leading edges of migrating mouse fibroblasts (Fig. 5g) and of migrating HUVECs (Fig. 5h). In conclusion, cPLA2α and phospho-S505-cPLA2α were found at protrusions of the cell membrane where actin remodeling takes place, indicating that cPLA2α activity plays an active role in this process.
Localization of phospho-S505-cPLA2α to circular dorsal ruffles and at leading edges. Relocalization of phospho-S505-cPLA2α to dorsal ruffles in C3H10T1/2 fibroblasts after PDGF stimulation. Fibroblasts were serum-deprived for 24 h and subsequently incubated in the presence (d–f) or absence (control a–c) of 20 ng/ml PDGF-BB for 15 min at 37°C. Cells were fixed and subjected to immunofluorescent staining (phospho-S505-cPLA2α green, F-actin red) and samples were analyzed by confocal microscopy. In non stimulated cells, staining for phospho-S505-cPLA2α is found in the cytoplasm and frequently in the nucleus (a) and F-actin is organized in large stress fibers (b). Stimulation with PDGF-BB results in the formation of circular dorsal ruffles (arrowhead) and the partial disappearance of stress fibers (e). Phospho-S505-cPLA2α translocates to the newly formed circular dorsal ruffles (d, arrowhead). Localization of phospho-S505-cPLA2α at the leading edges of migrating mouse fibroblasts and migrating HUVECs. Non-confluent mouse fibroblasts C3H10T1/2 (g) or HUVEC (h) were fixed and subjected to immunofluorescent staining with the anti-phospho-S505-cPLA2α antibody. Samples were analyzed by confocal microscopy. In both cell types, phospho-S505-cPLA2α is present at leading edges (arrowheads). Bar 10 μm
Inhibition of cPLA2α enzymatic activity prevents the formation of PDGF-induced circular ruffles and lamellae
To determine whether the enzymatic activity of cPLA2α is required for actin remodeling, we used the cPLA2α inhibitors Pyrrolidine-2 and Wyeth-1 characterized previously [30]. These non-structurally related inhibitors do not interfere with the enzymatic activity of calcium-independent PLA2 (iPLA2) or secreted PLA2s (sPLA2s) [30]. Serum-starved mouse fibroblasts were pre-treated with Pyrrolidine-2 or Wyeth-1 or mock-treated for 45 min. Pretreatment of cells with these inhibitors did not affect cellular morphology, nor did it affect the localization of cPLA2α compared to mock-treated cells (Fig. 6a, g). Subsequently, cells were treated for 15 min with 20 ng/ml PDGF-BB, they were fixed and F-actin was stained with phalloidin in parallel to cPLA2α immunodetection. Interestingly, when cells were pre-treated with either inhibitor, PDGF treatment did not induce circular dorsal ruffles nor lamellae (Fig. 6g–l), whereas in mock-treated cells, lamellae (data not shown) and dorsal ruffles containing cPLA2α were induced by PDGF (Fig. 6d–f, arrowheads). These data show that inhibition of cPLA2α enzymatic activity prevents PDGF-induced actin remodeling.
Inhibition of cPLA2α enzymatic activity with Pyrrolidine-2 or Wyeth-1 prevents PDGF-induced ruffling in C3H10T1/2 mouse fibroblasts. Fibroblasts were serum-deprived for 24 h and subsequently incubated in the absence (a–c) or presence (d–f) of 20 ng/ml PDGF-BB for 15 min at 37°C. Cells were fixed and subjected to immunofluorescent staining (cPLA2α red, F-actin green) and samples were analyzed by confocal microscopy. In non-stimulated cells cPLA2α is found in the cytoplasm (a) and F-actin is organized in large stress fibers (b). Incubation in the presence of PDGF results the partial disappearance of stress fibers and in the formation of membrane ruffles (e) to which cPLA2α translocates (d, arrowheads). Incubation of cells for 45 min with either Pyrrolidine-2 (g–i) or Wyeth-1 (j–l) before and during stimulation with PDGF prevents the formation of PDGF-induced dorsal ruffles. Bar 10 μm
Discussion
Arachidonic acid release has been demonstrated to play a role in actin polymerization. Since activation of cPLA2α results in the preferential release of arachidonic acid, it is tempting to suggest that cPLA2α is a key enzyme involved in the regulation of local actin dynamics. Therefore, we have studied the localization of cPLA2α in fibroblasts and HUVECs in relation to induced actin dynamics. Endogenous cPLA2α was shown to localize at the plasma membrane in both migrating mouse fibroblasts and migrating HUVEC. Moreover, the phosphorylated form of cPLA2α (on Ser505) was found at the plasma membrane in both cell types. Both cPLA2α and phospho-S505-cPLA2α localize at protrusions of the cell membrane that show active actin and membrane remodeling such as circular dorsal ruffles, lamellipodia, and leading edges. Interfering with the activity of cPLA2α in mouse fibroblasts resulted in the blocking of PDGF-induced actin and membrane dynamics.
cPLA2α is present at the cell membrane in a spatio-temporal manner. Upon PDGF stimulation, cPLA2α docks at specific sites at the cell membrane, namely at lamellipodia and ruffles. Upon stimulation of migration, cPLA2α docks at the leading edge of motile cells. Also here, the localization of cPLA2α at the cell membrane is local and temporal. This indicates that its docking at the cell membrane is tightly regulated. It was suggested that cPLA2α interacts with membranes via PtdIns(4,5)P2. It was shown that PtdIns(4,5)P2 stimulates cPLA2α activity independent of calcium suggesting an interaction between PtdIns(4,5)P2 and cPLA2α allowing cPLA2α to reach its substrate at membranes [31]. PtdIns(4,5)P2 is primarily located in the cytoplasmic leaflet of the plasma membrane. Interestingly, it has been demonstrated that a variety of signal transduction proteins, including growth factor receptors, reside in areas of high actin polymerization, such as membrane ruffles [7]. Receptor activation can increase or decrease local PtdIns(4,5)P2 concentrations via the local activation of kinases and phosphatases like PI5K, phospholipase C, phospholipase D, and PI3K [32]. By doing so, receptor activation might also regulate the docking of cPLA2α at the cell membrane upon growth factor stimulation. Interestingly PtdIns(4,5)P2 was shown to be enriched locally at the leading edge [33] and phosphatidylinositide metabolism was shown to be involved in ruffle formation [34–36].
The local presence of cPLA2α and its phosphorylated active form at sites of actin and membrane remodeling indicates a role for cPLA2α in regulating membrane dynamics. This is also suggested by blocking of PDGF-induced actin and membrane dynamics after exposure to the cPLA2α inhibitors Pyrrolidine-2 and Wyeth-1. Of note, these inhibitors also block the enzymatic activity of another cPLA2 from group IV, namely cPLA2ζ [30]. This enzyme can release arachidonic acid from membrane phospholipids but, contrary to cPLA2α, it does not have specificity for arachidonic acid versus other fatty acids [30]. However, in C3H10T1/2, cPLA2ζ is not expressed, as confirmed by RT-PCR (our unpublished data). This observation strengthens our inhibitors-based results, reinforcing the idea that cPLA2α plays a major role in regulating actin dynamics.
The local presence of the active form of cPLA2α might indicate a local production of arachidonic acid by cPLA2α. Subsequently, arachidonic acid might affect locally the behavior of actin via arachidonic metabolites. Arachidonic acid release has been previously related to the behavior of actin. During cell spreading, actin is changing the cytoskeleton and thereby changing the cell morphology. In HeLa cells, an increase in arachidonic acid production was shown after cell attachment. This results in both actin polymerization and actin bundling via two distinct pathways involving lipoxygenase and cyclooxygenase [9, 10]. Lipoxygenase metabolizes arachidonic acid and via a cascade of lipid second messengers protein kinase C epsilon (PKCε) is subsequently activated which triggers actin polymerization leading to cell spreading. Cyclooxygenase generates prostaglandins resulting in the activation of cyclic AMP-dependent protein kinase A (PKA) that induces actin bundling. Moreover, PKA was shown to play a role in the actin cytoskeleton regulation in a variety of ways (for review, see [37]). So, an increase of arachidonic acid causes an increase in actin polymerization and bundling during cell spreading by activating actin-binding proteins like PKA and PKCε. Furthermore, arachidonic acid and some of its metabolites can activate signaling pathways leading to the activation of small GTPases such as Rac1 and Rho [15, 16], ultimately resulting in actin remodeling. It has long been known that arachidonic acid and some of its metabolites can bind directly to and regulate the activity of specific GTPase-activating proteins (GAPs), which are regulators of small GTPases [38, 39]. Altogether, it is tempting to suggest that the local production of arachidonic acid and of its metabolites could trigger signaling pathways resulting in the local remodeling of actin.
In addition, cPLA2α is able to change the local characteristics of the plasma membrane by interacting with membrane phospholipids. cPLA2α-mediated hydrolysis of cellular membranes results in the production of lysophospholipids within the lipid bilayer and the lysophospholipids thus produced change the plasma membrane microviscosity. A change in the plasma membrane microviscosity by lysophospholipids was shown to affect cell migration [40]. Plasma membrane microviscosity has an important role on actin dynamics and thereby regulates cell motility [40, 41]. It was suggested that an increase in the plasma membrane microviscosity in lamellipodia might result in a local increase of actin-directed movement [41]. Our results show active cPLA2α at sites of active actin remodeling, and cPLA2α might here locally direct the behavior of actin by changing the plasma membrane microviscosity.
Altogether, our data show that cPLA2α is specifically recruited at sites of actin and membrane remodeling in different cellular processes. Since this phenomenon occurs in mouse fibroblasts and in primary human endothelial cells, it is likely to represent a general property of cPLA2α. However, further research is required to fully elucidate the role of cPLA2α in the above-mentioned processes.
Abbreviations
- PLA2 :
-
Phospholipase A2
- cPLA2α:
-
Cytosolic PLA2 alpha
- HUVECs:
-
Human umbilical vein endothelial cells
References
Boonstra J, Moes MJ (2005) Signal transduction and actin in the regulation of G1-phase progression. Crit Rev Eukaryot Gene Expr 15:255–276
Schleicher M, Jockusch BM (2008) Actin: its cumbersome pilgrimage through cellular compartments. Histochem Cell Biol 129:695–704
Naumanen P, Lappalainen P, Hotulainen P (2008) Mechanisms of actin stress fibre assembly. J Microsc 231:446–454
Prasain N, Stevens T (2009) The actin cytoskeleton in endothelial cell phenotypes. Microvasc Res 77:53–63
den Hartigh JC, en Henegouwen PM, Verkleij AJ, Boonstra J (1992) The EGF receptor is an actin-binding protein. J Cell Biol 119:349–355
Payrastre B, van Bergen en Henegouwen PM, Breton M, den Hartigh JC, Plantavid M, Verkleij AJ, Boonstra J (1991) Phosphoinositide kinase, diacylglycerol kinase, and phospholipase C activities associated to the cytoskeleton: effect of epidermal growth factor. J Cell Biol 115:121–128
Diakonova M, Payrastre B, van Velzen AG, Hage WJ, van Bergen en Henegouwen PM, Boonstra J, Cremers FF, Humbel BM (1995) Epidermal growth factor induces rapid and transient association of phospholipase C-gamma 1 with EGF-receptor and filamentous actin at membrane ruffles of A431 cells. J Cell Sci 108:2499–2509
Peppelenbosch MP, Tertoolen LG, Hage WJ, de Laat SW (1993) Epidermal growth factor-induced actin remodeling is regulated by 5-lipoxygenase and cyclooxygenase products. Cell 74:565–575
Stockton RA, Jacobson BS (2001) Modulation of cell-substrate adhesion by arachidonic acid: lipoxygenase regulates cell spreading and ERK1/2-inducible cyclooxygenase regulates cell migration in NIH-3T3 fibroblasts. Mol Biol Cell 12:1937–1956
Glenn HL, Jacobson BS (2002) Arachidonic acid signaling to the cytoskeleton: the role of cyclooxygenase and cyclic AMP-dependent protein kinase in actin bundling. Cell Motil Cytoskeleton 53:239–250
Panzer U, Uguccioni M (2004) Prostaglandin E2 modulates the functional responsiveness of human monocytes to chemokines. Eur J Immunol 34:3682–3689
Chen L, Iijima M, Tang M, Landree MA, Huang YE, Xiong Y, Iglesias PA, Devreotes PN (2007) PLA2 and PI3K/PTEN pathways act in parallel to mediate chemotaxis. Dev Cell 12:603–614
Navarro-Tito N, Robledo T, Salazar EP (2008) Arachidonic acid promotes FAK activation and migration in MDA-MB-231 breast cancer cells. Exp Cell Res 314:3340–3355
van Helden SF, Krooshoop DJ, Broers KC, Raymakers RA, Figdor CG, van Leeuwen FN (2006) A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J Immunol 177:1567–1574
Peppelenbosch MP, Qiu RG, de Vries-Smits AM, Tertoolen LG, de Laat SW, McCormick F, Hall A, Symons MH, Bos JL (1995) Rac mediates growth factor-induced arachidonic acid release. Cell 81:849–856
Shin EA, Kim KH, Han SI, Ha KS, Kim JH, Kang KI, Kim HD, Kang HS (1999) Arachidonic acid induces the activation of the stress-activated protein kinase, membrane ruffling and H2O2 production via a small GTPase Rac1. FEBS Lett 452:355–359
Schaloske RH, Dennis EA (2006) The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761:1246–1259
Boonstra J, van Rossum GS (2003) The role of cytosolic phospholipase A2 in cell cycle progression. Prog Cell Cycle Res 5:181–190
Jaffe EA, Nachman RL, Becker CG, Minick CR (1973) Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52:2745–2756
Buccione R, Orth JD, McNiven MA (2004) Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol 5:647–657
Rijken PJ, Post SM, Hage WJ, en Henegouwen PM, Verkleij AJ, Boonstra J (1995) Actin polymerization localizes to the activated epidermal growth factor receptor in the plasma membrane, independent of the cytosolic free calcium transient. Exp Cell Res 218:223–232
Schalkwijk CG, Spaargaren M, Defize LH, Verkleij AJ, van den Bosch H, Boonstra J (1995) Epidermal growth factor (EGF) induces serine phosphorylation-dependent activation and calcium-dependent translocation of the cytosolic phospholipase A2. Eur J Biochem 231:593–601
Spaargaren M, Wissink S, Defize LH, de Laat SW, Boonstra J (1992) Characterization and identification of an epidermal-growth-factor-activated phospholipase A2. Biochem J 287:37–43
van Rossum GS, Klooster R, van den Bosch H, Verkleij AJ, Boonstra J (2001) Phosphorylation of p42/44(MAPK) by various signal transduction pathways activates cytosolic phospholipase A(2) to variable degrees. J Biol Chem 276:28976–28983
Herbert SP, Ponnambalam S, Walker JH (2005) Cytosolic phospholipase A2-alpha mediates endothelial cell proliferation and is inactivated by association with the Golgi apparatus. Mol Biol Cell 16:3800–3809
Herbert SP, Odell AF, Ponnambalam S, Walker JH (2007) The confluence-dependent interaction of cytosolic phospholipase A2-alpha with annexin A1 regulates endothelial cell prostaglandin E2 generation. J Biol Chem 282:34468–34478
Grewal S, Morrison EE, Ponnambalam S, Walker JH (2002) Nuclear localisation of cytosolic phospholipase A2-alpha in the EA.hy.926 human endothelial cell line is proliferation dependent and modulated by phosphorylation. J Cell Sci 115:4533–4543
Grewal S, Ponnambalam S, Walker JH (2003) Association of cPLA2-alpha and COX-1 with the Golgi apparatus of A549 human lung epithelial cells. J Cell Sci 116:2303–2310
Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ (1993) cPLA2 is phosphorylated and activated by MAP kinase. Cell 72:269–278
Ghosh M, Loper R, Ghomashchi F, Tucker DE, Bonventre JV, Gelb MH, Leslie CC (2007) Function, activity, and membrane targeting of cytosolic phospholipase A(2)zeta in mouse lung fibroblasts. J Biol Chem 282:11676–11686
Balsinde J, Balboa MA, Li WH, Llopis J, Dennis EA (2000) Cellular regulation of cytosolic group IV phospholipase A2 by phosphatidylinositol bisphosphate levels. J Immunol 164:5398–5402
McLaughlin S, Murray D (2005) Plasma membrane phosphoinositide organization by protein electrostatics. Nature 438:605–611
Sharma VP, DesMarais V, Sumners C, Shaw G, Narang A (2008) Immunostaining evidence for PI(4, 5)P2 localization at the leading edge of chemoattractant-stimulated HL-60 cells. J Leukoc Biol 84:440–447
Araki N, Hatae T, Furukawa A, Swanson JA (2003) Phosphoinositide-3-kinase-independent contractile activities associated with Fc gamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci 116:247–257
Deming PB, Campbell SL, Baldor LC, Howe AK (2008) Protein kinase A regulates 3-phosphatidylinositide dynamics during platelet-derived growth factor-induced membrane ruffling and chemotaxis. J Biol Chem 283:35199–35211
Huang S, Lifshitz L, Patki-Kamath V, Tuft R, Fogarty K, Czech MP (2004) Phosphatidylinositol-4, 5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol Cell Biol 24:9102–9123
Howe AK (2004) Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta 1692:159–174
Tsai MH, Hall A, Stacey DW (1989) Inhibition by phospholipids of the interaction between R-ras, rho, and their GTPase-activating proteins. Mol Cell Biol 9:5260–5264
Yu CL, Tsai MH, Stacey DW (1990) Serum stimulation of NIH 3T3 cells induces the production of lipids able to inhibit GTPase-activating protein activity. Mol Cell Biol 10:6683–6689
Ghosh PK, Vasanji A, Murugesan G, Eppell SJ, Graham LM, Fox PL (2002) Membrane microviscosity regulates endothelial cell motility. Nat Cell Biol 4:894–900
Vasanji A, Ghosh PK, Graham LM, Eppell SJ, Fox PL (2004) Polarization of plasma membrane microviscosity during endothelial cell migration. Dev Cell 6:29–41
Acknowledgments
We like to thank Prof. M. Gelb for supplying the cPLA2α inhibitors. We thank C. Lenoir for supplying the GFP-cPLA2α constructs. We thank Bart de Haan and Miriam Langelaar-Makkinje for isolating HUVECs. We thank José Bijvelt and Mohammed El Khattabi for their help with the RT–PCR of cPLA2α and cPLA2ζ. This work is subsidized by the Dutch Space Organization (SRON, grant MG-059).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Moes, M., Boonstra, J. & Regan-Klapisz, E. Novel role of cPLA2α in membrane and actin dynamics. Cell. Mol. Life Sci. 67, 1547–1557 (2010). https://doi.org/10.1007/s00018-010-0267-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0267-0