Summary
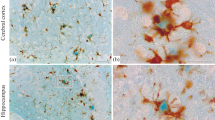
Ultrastructural studies of serial sections of the vessels with amyloid deposits in the brain cortex of patients with Alzheimer's disease showed that cells in the position of pericytes — perivascular cells - and perivascular microglial cells are producers of amyloid fibrils in the vascular wall. Three types of changes from normal are distinguishable in the vessel wall: (1) semicircular or circular thickening of vascular wall containing a large amount of amorphous material and various number of amyloid fibrils, (2) tuberous amyloid deposits containing both amorphous material and amyloid fibrils, some of the fibrils being arranged in strata and others arranged radially, and (3) amyloid star composed of a predominantly radial arrangement of bundles of amyloid fibrils and a less prominent amorphous component. A mixture of amorphous material and amyloid fibrils is present in cell membrane envaginations of perivascular cells, and occasionally perivascular microglial cells. Bundles of amyloid fibrils are found in altered cisternae of the endoplasmic reticulum and in the channels confluent with the infoldings of the plasma membrane of perivascular microglial cells. The amyloid deposition in the wall of the vessel causes degeneration of endothelial cells and the reduction of, and in some vessels obliteration of, the vessel lumen. In areas affected by amyloid angiopathy, extensive degeneration both of the neuropil and of neurons was observed. These changes were accompanied by astrogliosis. This study demonstrates similarities in amyloid formation in amyloid angiopathy and in β-amyloid plaques in the neuropil and suggests that cells of the mononuclear phagocyte system of the brain (perivascular cells and perivascular microglia) are engaged in amyloid fibril formation.
Similar content being viewed by others
References
Bartlett PF (1982) Pluripotential hemopoietic stem cells in adult mouse brain. Proc Natl Acad Sci USA 79:2722–2725
Castano EM, Frangione B (1988) Biology of disease. Human amyloidosis, Alzheimer disease and related disorders. Lab Invest 58:122–132
Cork LC, Masters C, Beyreuther K, Price DL (1990) Development of senile plaques. Relationships of neuronal abnormalities and amyloid deposits. Am J Pathol 137:1383–1392
Friede RL, Magee KR (1962) Alzheimer's disease: presentation of a case with pathologic and enzymatic-histochemical observations. Neurology 12:213–222
Fujita S, Tsuchihashi Y, Kitamura T (1981) Origin, morphology and function of the microglia. In: Glial and Neuronal Cell Biology. Eleventh International Congress of Anatomy. Alan R. Liss, New York, pp 141–169
Glenner GG, (1979) Congophilic microangiopathy in the pathogenesis of Alzheimer's syndrome (presenile dementia). Med Hypotheses 5:1231–1236
Glenner GG (1980) Amyloid deposits and amyloidosis. The β-fibrilloses. N Engl J Med 302:1283–1292, 1333–1343
Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Glenner GG, Wong CW (1984) Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122:1131–1135
Glenner GG, Wong CW (1986) The significance of cerebrovascular amyloid in Alzheimer's disease. International symposium on dementia and amyloid. Neuropathology [Suppl] 3:67–78
Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek CD (1987) Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer disease. Science 235:877–880
Graeber MB, Streit WJ (1990) Perivascular microglia defined. Trends Neurosci 13:66
Graeber MB, Streit WJ, Kreutzberg GW, (1989) Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res 22:103–106
Hart MN, Merz P, Bennett-Gray J, Menezes AH, Goeken JA, Schelper RL, Wisniewski HM (1988) β-Amyloid protein of Alzheimer's disease is found in cerebral and spinal cord vascular malformations. Am J Pathol 132:167–172
Hickey WF, Kimura H (1988) Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science 239:290–292
Ishihara T, Gondo T, Takahashi M, Uchino F, Ikeda S-I, Allsop D, Imai K (1991) Immunohistochemical and immunoelectron microscopical characterization of cerebrovascular and senile plaque amyloid in aged dog's brains. Brain Res 548:196–205
Ishii T (1958) Histochemistry of the senile changes of the brain of the senile dementia. Psychiatr Neurol Jpn 60:768–781
Ishii T (1969) Enzyme histochemical studies of senile plaques and the plaque-like degeneration of arteries and capillaries (Scholz). Acta neuropathol (Berl) 14:250–260
Kawai M, Kalaria RN, Harik SI, Perry G (1990) The relationship of amyloid plaques to cerebral capillaries in Alzheimer's disease. Am J Pathol 137:1435–1446
Mandybur TI (1975) The incidence of cerebral amyloid angiopathy in Alzheimer's disease. Neurology 25:120–126
Mandybur TI (1986) Cerebral amyloid angiopathy: the vascular pathology and complications. J Neuropathol Exp Neurol 45:79–90
Matsumoto Y, Kawai K, Fujiwara M (1989) In situ Ia expression on brain cells in the rat: autoimmune encephalomyelitis-resistant strain (BN) and susceptible strain (Lewis) compared. Immunology 66:621–627
Miyakawa T (1986) The relationship between amyloid fibrils around cerebral blood vessels and senile plaques, and ultrastructure of amyloid fibrils. International Symposium on Dementia and Amyloid. Neuropathology [Suppl] 3:37–48
Miyakawa T, Sumiyoshi S, Murayama E, Deshimaru M (1974) Ultrastructure of capillary plaque-like degeneration in senile dementia. Acta Neuropathol (Berl), 29:229–236
Miyakawa T, Shimoji A, Kumamoto R, Higuchi Y (1982) The relationship between senile plaques and cerebral blood vessels in Alzheimer's disease and senile dementia. Morphological mechanism of senile plaque production. Virchows Arch [B] 40:121–129
Morel F, Wildi E (1954) General and cellular pathochemistry of senile and presenile alterations of the brain. Proceedings of the 1st International Congress on Neuropathology, Rome, September 1952. Rosenberg and Sellier, Torino, pp 347–374
Morimatsu M, Hirai S, Muramatsu A, Yoshikawa M (1975) Senile degenerative brain lesions and dementia. J Am Geriatr Soc 23:390–406
Mountjoy CQ, Tomlinson BE, Gibson RH (1982) Amyloid and senile plaques and cerebral blood vessels. A semi-quantitative investigation of a possible relationship. J Neurol Sci 57:89–103
Osetowska E (1966) Etude anatomopatologique sur le cerveau de chiens senile. In: Luthy F, Bischoff A (eds) Proceedings of the Fifth International Congress of Neuropathology. Excerpta Medica, Amsterdam, pp 497–502
Peterson EW, Schulz DM, (1961) Amyloid in vessels of vascular malformations in brain. Arch Pathol 72:480–483
Robakis NK, Ramakrishna N, Wolfe G, Wisniewski HM, (1987) Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaques amyloid peptides. Proc Natl Acad Sci USA 84:4190–4194
Scholz W (1938) Studien zur Pathologie der Hirngefäße. II. Die drusige Entartung der Hirnarterien und Capillaren (eine Form seniler Gefäßerkrankung). Z Gesamte Neurol Psychiatr 162:694–715
Streit WJ, Graeber MB, Kreutzberg GW, (1989) Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol 105:115–126
Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, VanKeuran ML, Patterson D, Pagan S, Kurnit DM, Neve RL (1987) Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235:880–884
Tomonaga M (1981) Cerebral amyloid angiopathy in the elderly. J Am Geriatr Soc 29:151–157
van Duinen SG, Castano EM, Prelli F, Bots GTAB, Luyendijk W, Frangione B (1987) Hereditary cerebral hemorrhage with amyloidosis in patients of Dutch origin is related to Alzheimer's disease. Proc Natl Acad Sci USA 84:5991–5994
Vinters HV, Gilbert JJ (1983) Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke 14:924–928
Vinters HV, Miller BL, Pardrige WM, (1988) Brain amyloid and Alzheimer disease. Ann Intern Med 109:41–54
von Braunmuhl A (1956) Kongophile Angiopathie und senile Plaques bei greisen Hunden. Arch Psychiatr Nervenkr 194:396–414
Walker LC, Masters C, Beyreuther K, Price DL (1990) Amyloid in the brains of aged squirrel monkeys. Acta Neuropathol 80:381–387
Wegiel J, Wisniewski HM (1990) The complex of microglial cells and amyloid star in three-dimensional reconstruction. Acta Neuropathol 81:116–124
Wisniewski HM, Terry RD (1973) Reexamination of the pathogenesis of the senile plaque. Prog Neuropathol 2:1–26
Wisniewski HM, Wegiel J (1991) Spatial relationships between astrocytes and classical plaque components. Neurobiol Aging 12:593–600
Wisniewski HM, Johnson AB, Raine CS, Kay WJ, Terry RD (1970) Senile plaques and cerebral amyloidosis in aged dogs. A histochemical and ultrastructural study. Lab Invest 23:287–296
Wisniewski HM, Wegiel J, Wang KC, Kujawa M, Lach B (1989) Ultrastructural studies of the cells forming amyloid fibers in clasical plaques. Can J Neurol Sci 16:535–542
Wisniewski HM, Wegiel J, Morys J, Bancher C, Soltysiak Z, Kim KS (1990) Aged dogs: an animal model to study beta-protein amyloidogenesis. In: Maurer K, Riederer P, Beckmann H (eds) Alzheimer's disease. Epidemiology, neuropathology, neurochemistry, and clinics (Key topics in brain research). Springer Verlag, Wien New York, pp 151–168
Wisniewski HM, Wegiel J, Strojny P, Wang K-C, Kim K-S, Burrage T (1991) Ultrastructural morphology and immunocytochemistry of beta amyloid classical, primitive and diffuse plaque. In: Ishii T, et al (eds) Frontiers of Alzheimer research. Elsevier, Amsterdam, pp 99–108
Wisniewski HM, Weigiel J, Vorbrodt AW, Barcikowska M, Burrage TG (1991) The cellular basis for β-amyloid fibril formation and removal. In: Iqbal K, McLachlan DRC, Winblad B,Wisniewski HM (eds) Alzheimer's disease: basic mechanisms, diagnosis and therapeutic strategies. John Wiley and Sons, Chichester, pp 333–339
Author information
Authors and Affiliations
Additional information
Supported in part by funds from the New York State Office of Mental Retardation and Developmental Disabilities and a grant from the National Institutes of Health, National Institute of Aging No. PO1-AGO-4220
Rights and permissions
About this article
Cite this article
Wisniewski, H.M., Wegiel, J., Wang, K.C. et al. Ultrastructural studies of the cells forming amyloid in the cortical vessel wall in Alzheimer's disease. Acta Neuropathol 84, 117–127 (1992). https://doi.org/10.1007/BF00311383
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00311383