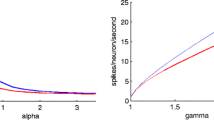
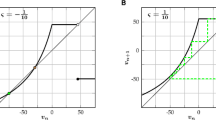
Abstract
Feature linking and pattern separation are shown to be performed as simultaneous processes by a highly connected auto-associative network of spiking neurons (spike response model). In principle, many (e.g., with nine) patterns can be separated, but with a biological set of parameters the number is limited to four. The patterns have been learned by an asymmetric hebbian rule that can handle a low activity which may vary from pattern to pattern (in a range between 4% and 7%). Spikes are generated by a threshold process and with some delay transmitted to postsynaptic neurons. There they evoke an excitatory or inhibitory postsynaptic potential (EPSP or IPSP). Spike emission is followed by an absolute refractory period (1 ms) and activates an inhibitory delay loop that prevents continuous firing. Three different network topologies are discussed, i.e., a structureless fully connected system, a network composed of two ‘hemispheres’, and finally a hierarchical network with four subsystems that represent different ‘functions’ and interact via feedforward and feedback connections. Functional feedback turns out to be essential for context-sensitive binding. The coherence between the two hemispheres is dependent on the interhemispheric delays. If these are on average too large, the two hemispheres oscillate coherently by themselves but phase-shifted by half a period with respect to each other.
Similar content being viewed by others
References
Biedermann J (1990) Higher-level vision. In: Visual cognition and action. Osherson DN, Kosslyn SM, Hollerbach JM (eds) MIT Press, Cambridge, Mass., pp 41–72
Buhmann J (1989) Oscillations and low firing rates in associative memory neural networks. Phys Rev A 40:4145–4148
Eckhorn R, Brosch M (1993) Synchronous oscillatory activities between areas 17 and 18 in the cat's visual cortex. Preprint
Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ (1988) Coherent oscillations: a mechanism of feature linking in the visual cortex? Biol Cybern 60:121–130
Eckhorn R, Frien A, Bauer R, Woelbern T, Kehr H (1993) High frequency (60–90 Hz) oscillations in primary visual cortex of awake monkey. Neuro Rep 4:243–246
Engel AK, König P, Kreiter K, Singer W (1991a) Interhemispheric synchronization of oscillatory neural responses in cat visual cortex. Science 252:1177–1179
Engel AK, König P, Singer W (1991b) Direct physiological evidence for scene segmentation by temporal coding. Proc Natl Acad Sci USA 88:9136–9140
Engel AK, Kreiter K, König P, Singer W (1991c) Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proc Natl Acad Sci USA 88:6048–6052
Felleman DJ, van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47
Fuentes U (1993) Einfluß der Schichtund Arealstruktur auf die Informationsverarbeitung im Cortex. Diplomarbeit, Technische Universität München
Gerstner W, Ritz R, van Hemmen JL (1993a) A biologically motivated and analytically soluble model of collective oscillations in the cortex. I. Theory of weak locking. Biol Cybern 68:363–374
Gerstner W, Ritz R, van Hemmen JL (1993b) Why spikes? Hebbian learning and retrieval of time-resolved excitation patterns. Biol Cybern 69:503–515
Gray CM, Singer W (1989) Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA 86:1698–1702
Gray CM, König P, Engel AK, Singer W (1989) Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338:334–337
Hebb DO (1949) The organization of behavior. Wiley, New York
Hemmen JL van, Gerstner W, Herz AVM, Kühn R, Sulzer B, Vaas M (1990) Encoding and decoding of patterns which are correlated in space and time. In:Dorffner G (ed) Konnektionismus in Artificial Intelligence und Kognitionsforschung. Springer, Berlin Heidelberg New York, pp 153–162
Hopfield JJ (1982) Neural networks and physical systems with emergent collective computational abilities. Proc Natl Acad Sci USA 79:2554–2558
Horn D, Usher M (1991) Parallel activation of memories in an oscillatory neural network. Neural Comput 3:31–43
Horn D, Usher M (1992) Oscillatory model of short term memory. In: Moody JE, Hanson SJ, Lippmann RP (eds) Advances in Neural Information Processing Systems, vol 4. Morgan Kaufmann, San Mateo, pp 125–132
Horn D, Sagi D, Usher M (1991) Segmentation, binding, and illusory conjunctions. Neural Comput 3:510–525
König P, Schulen TB (1991) Stimulus-dependent assembly formation of oscillatory responses. I. Synchronization. Neural Comput 3:155–166
Kreiter AK, Singer W (1992) Oscillatory neuronal responses in the visual cortex of the awake macaque monkey. Eur J Neurosci 4:369–375
Kuramoto Y (1984) Chemical oscillations, waves, and turbulence. Springer, Berlin Heidelberg New York, pp 68–77
Malsburg C von der (1981) The correlation theory of brain function. (Internal Report 81-2) MPI für Biophysikalische Chemie, Göttingen
Malsburg C von der, Buhmann J (1992) Sensory segmentation with coupled neural oscillators. Biol Cybern 67:233–242
Malsburg C von der, Schneider W (1986) A neural cocktail-party processor. Biol Cybern 54:29–40
Neven H, Aertsen A (1992) Rate coherence and event coherence in the visual cortex: a neural model of object recognition. Biol Cybern 67:309–322
Schillen TB, König P (1991) Stimulus-dependent assembly formation of oscillatory responses. II. Desynchronization. Neural Comput 3:167–178
Sperling G (1960) The information available in brief visual presentations. Psychol Monogr 74 (11 Whole No. 498):1–29
Sporns O, Gally JA, Reeke GN, Edelman GM (1989) Reentrant signaling among simulated neural groups leads to coherency in their oscillatory activity. Proc Natl Acad Sci USA 86:7265–7269
Sporns O, Tononi G, Edelman GM (1991) Modeling perceptual grouping and figure-ground segregation by means of active reentrant connections. Proc Natl Acad Sci USA 88:129–133
Sutherland S (1991) Only four possible solutions. Nature 353:389–390
Sutton JP, Beis JS, Trainor LEH (1988) Hierarchical model of memory and memory loss. J Phys A 21:4443–4454
Trefz T (1991) Oszillationen im Cortex. Diplomarbeit, Technische Universität München
Wang D, Buhmann J, von der Malsburg C (1990) Pattern segmentation in associative memory. Neural Comput 2:94–106
Zeki S, Shipp S (1988) The functional logic of cortical connections. Nature 335:310–317
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ritz, R., Gerstner, W., Fuentes, U. et al. A biologically motivated and analytically soluble model of collective oscillations in the cortex. Biol. Cybern. 71, 349–358 (1994). https://doi.org/10.1007/BF00239622
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00239622