Abstract
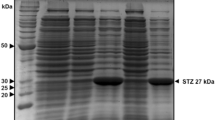
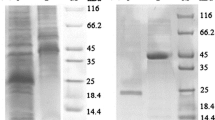
A gene from groundnut (Arachis hypogaea) coding for stilbene synthase was transferred together with a chimaeric kanamycin resistance gene. It was found to be rapidly expressed after induction with UV light and elicitor in tobacco cells (Nicotiana tabacum). Comparative studies of stilbene synthase mRNA synthesis in groudnut and transgenic tobacco suspension cultures revealed the same kinetics of gene expression. Stilbene synthase specific mRNA was detectable 30 minutes after elicitor induction and 10 minutes after UV irradiation. The maximum of mRNA accumulation was between 2 and 8 hours post induction. 24 hours after induction stilbene synthase mRNA accumulation ceased. Furthermore, in transgenic tobacco plants, the gene was found to be inducible in sterile roots, stems and leaves. Stilbene synthase was demonstrated in crude protein extracts from transgenic tobacco cell cultures using specific antibodies. Resveratrol, the product of stilbene synthase, was identified by HPLC and antisera raised against resveratrol.
Similar content being viewed by others
References
Bell AA: Biochemical mechanisms of disease resistance. Ann Rev Plant Physiol 32: 21–81 (1981).
Buchmann I, Marner FJ, Schröder G, Waffenschmidt S, Schröder J: Tumour genes in plants: T-DNA encoded cytokinin biosynthesis. EMBO J 4: 853–859 (1985).
Chappel J, Hahlbrock K: Transcription of plant defense genes in response to UV light or fungal elicitor. Nature 311: 76–78 (1984).
Ebel J: Phytoalexin synthesis: The biochemical analysis of the induction process. Ann Rev Phytopath 24: 235–264 (1986).
Gorham J: The stilbenoids. Progr Phytochem 6: 203–252 (1980).
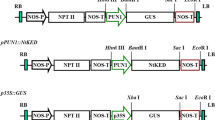
Hain R, Stabel P, Czernilofsky AP, Steinbiss HH, Herrera-Estrella L, Schell J: Uptake integration and expression and genetic transmission of a selectable chimaeric gene by plant protoplasts. Mol Gen Genet 199: 161–168 (1985).
Hart JH: Role of phytostilbenes in decay and disease resistance. Ann Rev Phytopath 19: 437–458 (1981).
Hurn BAL, Chantler SM: Production of reagent antibodies. Meth Enzymol 70: 104 (1980).
Ingham JL: 3,5,4 trihydroxystilbene as a phytoalexin from groundnuts (Arachis hypogaea). Phytochemistry 15: 1791–1793 (1976).
Jahnen W, Hahlbrock K: Cellular localization of nonhost resistance reactions of parsley (Petroselinum crispum) to fungal infection. Planta 173: 197–204 (1988).
Kobrink E, Schröder M, Hahlbrock K: Several ‘pathogenesis-related’ proteins in potatoes are 1,3-β-glucanases and chitinases. Proc Natl Acad Sci USA 84: 782–786 (1988).
Linsmaier EM, Skoog F: Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18: 100–127 (1965).
Maliga P, Sz.-Breznovitis, Marton L: Streptomycin-resistant plants from callus culture of haploid tobacco. Nature, New Biol 244: 29–30 (1973).
Maniatis T, Fritsch EF, Sambrook J: Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY (1982).
Murashige T, Skoog F: A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15: 473–497 (1962).
Rolfs CH, Fritzemeier KH, Kindl H: Cultured cells of Arachis hypogaea susceptible to induction of stilbene synthase (resveratrol forming). Plant Cell Rep 1: 83–85 (1981).
Rolfs CH, Schön H, Steffens M, Kindl H: Cell suspension culture of Arachis hypogaea: Model system of specific enzyme induction in secondary metabolism. Planta 172: 238–244 (1987).
Rupprich N, Kindl H: Stilbene synthases and stilbene carboxylate synthases, I. Enzymatic syntheses of 3,5,4′-trihydroxystilbene from p-coumaroyl CoA and malonyl CoA. Hoppe-Seyler's Z Physiol Chem 359: 165–172 (1978).
Ryder TB, Cramer CR, Bell JN, Robbins MP, Dixon RA, Lamb JC: Elicitor rapidly induces chalcone synthase mRNA in Phaseolus vulgaris cells at the onset of the phytoalexin defense response. Proc Natl Acad Sci USA 81: 5724–5728 (1984).
Salt GA: The increasing interest in ‘minor pathogens’. In: Schippers B, Gams W: Soil-borne Plant Pathogens. Academic Press, London, New York, San Francisco (1979).
Scheel D, Hauffe KD, Jahnen W, Hahlbrock K: Stimulation of phytoalexin formation in fungus-infected plants and elicitor-treated cell cultures of parsley. In: Lugtenberg B (ed) NATO ASI Series, H4: 325–331 (1986).
Schenk R, Hildebrand C: Medium and techniques for the induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50: 199–204 (1972).
Schöppner A, Kindl H: Purification and properties of a stilbene synthase from induced cell suspension cultures of peanut. J Biol Chem 259: 6806–6811 (1984).
Schröder G, Brown JWS, Schröder J: Molecular analysis of resveratrol synthase: cDNA, genomic clones and relationship with chalconsynthase. Eur J Biochem 172: 161–169 (1988).
Schröder J, Lanz T, Schröder G: Genes for biosynthesis of stilbene-type phytoalexins. In: Lamb C, Beachy R (eds) Plant Gene Transfer, p. 129. A.R. Liss, Inc, New York, in press (1989).
Sequeira L: Mechanisms of induced resistance in plants. Ann Rev Microbiol 37: 51–79 (1983).
Stein U, Blaich R: Untersuchungen über Stilbenproduktion und Botrytisanfälligkeit bei Vitis-Arten. Vitis 24: 75–87 (1985).
Stöcker RH: Immunologische Analysen zur Reduktion des Fungizids Triadimefon. Ph.D. thesis, University of Bochum, FRG (1985).
Vornam B, Schön H, Kindl H: Control of gene expression during induction of cultured peanut cells: mRNA levels, protein synthesis and enzyme activity of stilbene synthase. Plant Mol Biol 10: 235–243 (1988).
Weiler EW: Radioimmunoassays for the differential and direct analysis of free and conjugated abscisic acid in plant extracts. Planta 148: 262 (1980).
Wilson MB, Nakane PK: In: Immunofluorescence and Related Techniques, p. 215. Elsevier, Amsterdam (1978).
Woodward S, Pearce RB: The role of stilbenes in resistance of Sitka spruce (Picea sitchensis) (Bong.) Carr. to entry of fungal pathogens. Physiol Mol Plant Path 33: 127–149 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hain, R., Bieseler, B., Kindl, H. et al. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol Biol 15, 325–335 (1990). https://doi.org/10.1007/BF00036918
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00036918