Abstract
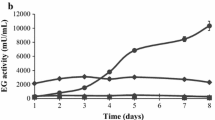
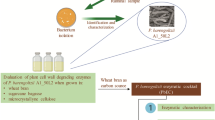
The microorganisms living on the phyllosphere (the aerial part of the plants) are in contact with the lignocellulosic plant cell wall and might have a lignocellulolytic potential. We isolated a Saccharibacillus strain (Saccharibacillus WB17) from wheat bran phyllosphere and its cellulolytic and hemicellulolytic potential was investigated during growth onto wheat bran. Five other type strains from that genus selected from databases were also cultivated onto wheat bran and glucose. Studying the chemical composition of wheat bran residues by FTIR after growth of the six strains showed an important attack of the stretching C-O vibrations assigned to polysaccharides for all the strains, whereas the C = O bond/esterified carboxyl groups were not impacted. The genomic content of the strains showed that they harbored several CAZymes (comprised between 196 and 276) and possessed four of the fifth modules reflecting the presence of a high diversity of enzymes families. Xylanase and amylase activities were the most active enzymes with values reaching more than 4746 ± 1400 mIU/mg protein for the xylanase activity in case of Saccharibacillus deserti KCTC 33693 T and 452 ± 110 mIU/mg protein for the amylase activity of Saccharibacillus WB17. The total enzymatic activities obtained was not correlated to the total abundance of CAZyme along that genus. The Saccharibacillus strains harbor also some promising proteins in the GH30 and GH109 modules with potential arabinofuranosidase and oxidoreductase activities. Overall, the genus Saccharibacillus and more specifically the Saccharibacillus WB17 strain represent biological tools of interest for further biotechnological applications.




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Kim JY, Lee HW, Lee SM, Jae J, Park YK (2019) Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour Technol 279:373–384
Viikari L, Vehmaanperä J, Koivula A (2012) Lignocellulosic ethanol: from science to industry. Biomass Bioenerg 46:13–24
Chandel AK et al (2018) The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Bioresour Technol 264:370–381
FitzPatrick M et al (2010) A biorefinery processing perspective: treatment of lignocellulosic materials for the production of value-added products. Bioresour Technol 101(23):8915–8922
Sun R-C (2009) Detoxification and separation of lignocellulosic biomass prior to fermentation for bioethanol production by removal of lignin and hemicelluloses. BioResources 4(2):452–455
Liu C et al (2017) Valorization of untreated rice bran towards bioflocculant using a lignocellulose-degrading strain and its use in microalgal biomass harvest. Biotechnol Biofuels 10(1):90
Martinez D et al (2009) Genome, transcriptome, and secretome analysis of wood decay fungus Postia placenta supports unique mechanisms of lignocellulose conversion. Proc Natl Acad Sci 106(6):1954–1959
Prueckler M et al (2014) Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT-Food Sci Technol 56(2):211–221
Kim S, Dale BE (2004) Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenerg 26(4):361–375
McCann MC, Carpita NC (2015) Biomass recalcitrance: a multi-scale, multi-factor, and conversion-specific property. J Exp Bot 66(14):4109–4118
Amarasekara AS (2013) Handbook of cellulosic ethanol. John Wiley & Sons
Zhao X, Zhang L, Liu D (2012) Biomass recalcitrance Part I:the chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod Biorefin 6(4):465–482
Wilhelm RC et al (2019) Bacterial contributions to delignification and lignocellulose degradation in forest soils with metagenomic and quantitative stable isotope probing. ISME J 13(2):413–429
Gontikaki E, Thornton B, Cornulier T, Witte U (2015) Occurrence of priming in the degradation of lignocellulose in marine sediments. PLoS One 10(12):e0143917
Luo C et al (2019) Bamboo lignocellulose degradation by gut symbiotic microbiota of the bamboo snout beetle Cyrtotrachelus buqueti. Biotechnol Biofuels 12(1):70
Vorholt JA (2012) Microbial life in the phyllosphere. Nat Rev Microbiol 10(12):828–840
Pati B, Sengupta S, Chandra A (1995) Role of nitrogen fixing bacteria on the phyllosphere of wheat seedlings. Acta Microbiol Immunol Hung 42(4):427–433
Mwajita MR et al (2013) Evaluation of rhizosphere, rhizoplane and phyllosphere bacteria and fungi isolated from rice in Kenya for plant growth promoters. Springerplus 2(1):606
Knorr K, Jørgensen LN, Nicolaisen M (2019) Fungicides have complex effects on the wheat phyllosphere mycobiome. PLoS ONE 14(3):e0213176
Besaury L, Remond C (2020) Draft genome sequence of saccharibacillus sp. strain WB 17, Isolated from Wheat Phyllosphere. Microbiol Resour Announc 9(7):e01201–e01219
Rivas R et al (2008) Saccharibacillus sacchari gen. nov. sp. nov. isolated from sugar cane. Int J Syst Evol Microbiol 58(PT8):1850–4
Sun JQ et al (2016) Saccharibacillus deserti sp nov isolated from desert soil. Int J Syst Evol Microbiol 66(2):623–627
Yang SY et al (2009) Saccharibacillus kuerlensis sp nov isolated from a desert soil. Int J Syst Evol Microbiol 59(5):953–7
Han H et al (2016) Saccharibacillus qingshengii sp nov isolated from a lead-cadmium tailing. Int J Syst Evol Microbiol 66(11):4645–4649
Kampfer P et al (2016) Saccharibacillus endophyticus sp nov an endophyte of cotton. Int J Syst Evol Microbiol 66(12):5134–5139
De Vos P, Ludwig W, Schleifer KH, Whitman WB (2011) Family IV. Paenibacillaceae fam. nov. Bergey's Manual of Systematic Bacteriology 3, 269
Weselowski B et al (2016) Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiol 16(1):244
Pontonio E et al (2018) Dynamic and assembly of epiphyte and endophyte lactic acid bacteria during the life cycle of Origanum vulgare L. Front Microbiol 9:1372
Yang L et al (2017) Dominant groups of potentially active bacteria shared by barley seeds become less abundant in root associated microbiome. Front Plant Sci 8:1005
Pswarayi F, Gänzle MG (2019) Composition and origin of the fermentation microbiota of mahewu, a Zimbabwean fermented cereal beverage. Appl Environ Microbiol 85(11):e03130–18
Tanca A et al (2017) Metaproteogenomics reveals taxonomic and functional changes between cecal and fecal microbiota in mouse. Front Microbiol 8:391
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. Babraham Bioinformatics, Babraham Institute, Cambridge, United Kingdom
Peng Y et al (2012) IDBA-UD: a de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 28(11):1420–1428
Parks DH et al (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25(7):1043–1055
Karp PD et al (2002) The metacyc database. Nucleic Acids Res 30(1):59–61
Yin Y et al (2012) dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res 40(W1):W445–W451
Armenteros JJA et al (2019) SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat Biotechnol 37(4):420–423
Krogh A et al (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305(3):567–580
Kidby D, Davidson D (1973) A convenient ferricyanide estimation of reducing sugars in the nanomole range. Anal Biochem 55(1):321–325
Rakotoarivonina H et al (2012) The hemicellulolytic enzyme arsenal of Thermobacillus xylanilyticus depends on the composition of biomass used for growth. Microb Cell Fact 11(1):159
Puentes-Téllez PE, Salles JF (2018) Construction of effective minimal active microbial consortia for lignocellulose degradation. Microb Ecol 76(2):419–429
Metsalu T, Vilo J (2015) ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res 43(W1):W566–W570
Poria V, Saini JK, Singh S, Nain L, Kuhad RC (2020) Arabinofuranosidases: characteristics, microbial production, and potential in waste valorization and industrial applications. Bioresour Technol 304:123019
Bouraoui H et al (2016) The GH51 α-L-arabinofuranosidase from Paenibacillus sp THS1 is multifunctional hydrolyzing main-chain and side-chain glycosidic bonds in heteroxylans. Biotechnol Biofuels 9(1):1–14
Bohra V, Tikariha H, Dafale NA (2019) Genomically defined Paenibacillus polymyxa ND24 for efficient cellulase production utilizing sugarcane bagasse as a substrate. Appl Biochem Biotechnol 187(1):266–281
Gangoiti J et al (2017) Characterization of the Paenibacillus beijingensis DSM 24997 GtfD and its glucan polymer products representing a new glycoside hydrolase 70 subfamily of 4, 6-α-glucanotransferase enzymes. PLoS ONE 12(4):e0172622
Maeng S et al (2019) Cohnella candidum sp nov radiation-resistant bacterium from soil. Antonie Van Leeuwenhoek 112(7):1029–1037
Jiang L et al (2019) Cohnella abietis sp nov isolated from Korean fir (Abies koreana) rhizospheric soil of Halla mountain. J Microbiol 57(11):953–958
Chao L, Jongkees S (2019) High-Throughput Approaches in Carbohydrate-Active Enzymology: glycosidase and glycosyl transferase inhibitors, evolution, and discovery. Angew Chem Int Ed Engl 131(37):12880–12890
Spertino S et al (2018) Cellulomonas fimi secretomes: in vivo and in silico approaches for the lignocellulose bioconversion. J Biotechnol 270:21–29
Granja-Travez RS, Bugg TD (2018) Characterization of multicopper oxidase CopA from Pseudomonas putida KT2440 and Pseudomonas fluorescens Pf-5: Involvement in bacterial lignin oxidation. Arch Biochem Biophys 660:97–107
Forney LJ et al (1982) The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem 257(19):11455–11462
Martínez A et al (2018) Biological lignin degradation. Lignin Valorization: Emerging Approaches 19:199
Westermark U, Eriksson KE (1974) Carbohydrate-dependent enzymic quinone reduction during lignin degradation. Acta chem scand B 28(2):204–208
Kontur WS et al (2018) Novosphingobium aromaticivorans uses a Nu-class glutathione S-transferase as a glutathione lyase in breaking the β-aryl ether bond of lignin. J Biol Chem 293(14):4955–4968
Adesioye FA et al (2016) Phylogeny, classification and metagenomic bioprospecting of microbial acetyl xylan esterases. Enzyme Microb Technol 93:79–91
Zheng H-C et al (2014) Purification and characterization of a thermostable xylanase from Paenibacillus sp NF1 and its application in xylooligosaccharides production. J microbiol biotechnol 24(4):489–496
Tsusaki K, Watanabe H, Yamamoto T, Nishimoto T, Chaen H, Fukuda S (2012) Purification and characterization of highly branched α-glucan–producing enzymes from Paenibacillus sp. PP710. Biosci Biotechnol Biochem 1203292865–1203292865
Ikram-Ul-Haq HU, Mahmood Z, Javed MM (2012) Solid state fermentation for the production of α-amylase by Paenibacillus amylolyticus. Pak J Bot 44:341–346
Rajesh T et al (2013) Identification and Functional Characterization of an α-Amylase with Broad Temperature and pH Stability from Paenibacillus sp. Appl Biochem Biotechnol 170(2):359–369
Zhang XZ, Zhang YHP (2010) One-step production of biocommodities from lignocellulosic biomass by recombinant cellulolytic Bacillus subtilis: Opportunities and challenges. Eng Life Sci 10(5):398–406
Waghmare PR et al (2014) Enzymatic hydrolysis and characterization of waste lignocellulosic biomass produced after dye bioremediation under solid state fermentation. Bioresour Technol 168:136–141
Sills DL, Gossett JM (2012) Using FTIR spectroscopy to model alkaline pretreatment and enzymatic saccharification of six lignocellulosic biomasses. Biotechnol Bioeng 109(4):894–903
Heidary Vinche M, Khanahmadi M, Ataei SA, Danafar F (2021) Optimization of process variables for production of beta-glucanase by Aspergillus niger CCUG33991 in solid-state fermentation using wheat bran. Waste Biomass Valoriz 12(6):3233–3243
Fackler K, Stevanic JS, Ters T, Hinterstoisser B, Schwanninger M, Salmén L (2011) FT-IR imaging microscopy to localise and characterise simultaneous and selective white-rot decay within spruce wood cells
Xu F et al (2013) Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: a mini-review. Appl Energy 104:801–809
Lun LW, Gunny AAN, Kasim FH, Arbain D (2017) Fourier transform infrared spectroscopy (FTIR) analysis of paddy straw pulp treated using deep eutectic solvent. In AIP conference proceedings, vol 1835, no 1. AIP Publishing LLC, p 020049
Wang Z et al (2013) The effects of ultrasonic/microwave assisted treatment on the properties of soy protein isolate/microcrystalline wheat-bran cellulose film. J Food Eng 114(2):183–191
Wilson CM et al (2017) LacI transcriptional regulatory networks in Clostridium thermocellum DSM1313. Appl Environ Microbiol 83(5):e02751-e2816
Bai W et al (2016) Improvement of alkalophilicity of an alkaline xylanase Xyn11A-LC from Bacillus sp SN5 by random mutation and Glu135 saturation mutagenesis. BMC Biotechnol 16(1):1–9
Ghazi S et al (2014) UV mutagenesis for the overproduction of xylanase from Bacillus mojavensis PTCC 1723 and optimization of the production condition. Iran J Basic Med Sci 17(11):844
Dutta B et al (2018) In silico studies on bacterial xylanase enzyme: structural and functional insight. J Genet Eng Biotechnol 16(2):749–756
Liu G et al (2013) Genomic and secretomic analyses reveal unique features of the lignocellulolytic enzyme system of Penicillium decumbens. PLoS ONE 8(2):e55185
Shi H et al (2014) Characterization of a novel GH2 family α-L-arabinofuranosidase from hyperthermophilic bacterium Thermotoga thermarum. Biotech Lett 36(6):1321–1328
Matsuo N et al (2000) Purification, characterization and gene cloning of two α-L-arabinofuranosidases from Streptomyces chartreusis GS901. Biochem J 346(1):9–15
Miyazaki K (2005) Hyperthermophilic α-L-arabinofuranosidase from Thermotoga maritima MSB8: molecular cloning, gene expression, and characterization of the recombinant protein. Extremophiles 9(5):399–406
Chacón-Martínez CA et al (2004) Identification and characterization of the α-l-arabinofuranosidase B of Fusarium oxysporum f sp dianthi. Physiol Mol Plant Pathol 64(4):201–208
Tsujibo H et al (2002) Cloning and expression of an α-L-arabinofuranosidase gene (stxIV) from Streptomyces thermoviolaceus OPC-520, and characterization of the enzyme. Biosci Biotechnol Biochem 66(2):434–438
Zhou J et al (2012) Biochemical and kinetic characterization of GH43 β-D-xylosidase/α-L-arabinofuranosidase and GH30 α-L-arabinofuranosidase/β-D-xylosidase from rumen metagenome. J Ind Microbiol Biotechnol 39(1):143–152
Jones P et al (2014) InterProScan 5: genome-scale protein function classification. Bioinformatics 30(9):1236–1240
Acknowledgements
This study was funded by the project ANS Fralicocmi supported by the CEPIA Department from the French National Institute for Agricultural Research and Environment (INRAE). The authors are grateful to the French Region Grand Est, Grand Reims and the European Regional Development Fund (ERDF) for the financial support of the chaire AFERE.
Author information
Authors and Affiliations
Contributions
Ludovic Besaury: investigation, supervision, methodology, original draft preparation, reviewing and editing; Mathilde Bocquart: investigation; Caroline Remond: reviewing and editing.
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luiz Henrique Rosa.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Besaury, L., Bocquart, M. & Rémond, C. Isolation of Saccharibacillus WB17 strain from wheat bran phyllosphere and genomic insight into the cellulolytic and hemicellulolytic complex of the Saccharibacillus genus. Braz J Microbiol 53, 1829–1842 (2022). https://doi.org/10.1007/s42770-022-00819-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-022-00819-w