Abstract
Core–shell YFeO3@Al2O3 structure was obtained using advanced synthesis process. YFeO3@Al2O3 assemble was achieved by coating individual YFeO3 particles with several nanometers thickness of alumina-shell. After that, the particles characterization and the thickness control of the magnetic core/alumina interface were investigated by X-ray diffraction, scanning electron microscopy and transmission electron microscopy. Finally, the magnetic properties of the resulting system were investigated. We found that the alumina-shell thickness diminishes the magnetization values and enhance the exchange bias effect at low magnetic field. This fact is explained by the pinning the weak ferromagnetic and antiferromagnetic domains wall motion at the interface of the core/shell during the descending and ascending magnetization in the hysteresis curves. The results prove that the weak-ferromagnetic and the antiferromagnetic ordering not only coexist but also they are weakly coupled in YFeO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, considerable effort has been devoted to designing and to control the fabrication of nanocomposite materials with functional properties [1]. Core–shell architecture is an effective strategy to build tailored nanomaterials, which are of great interest from fundamental, academic and technological points of view [2]. The core–shell approach often exhibits different physical and chemical properties from the single-component counterparts [2, 3]. This architecture calls for strict control of the chemical composition, particle size, interfaces reactivity and excellent control of the thickness of the shell. Atomic layer deposition (ALD) has been used to fabricate powder nanocomposite with core shell structure. By this technique, it is possible to attain a uniform and complete control of the shell-thickness [4,5,6,7]. Recently, magnetic nanocomposites have attracted particular attention due to their potential application as microwave absorbing materials and electromagnetic devices [8]. For example, nanocomposites with soft magnetic properties can be used in high-frequency devices requiring low coercivity and high resistivity. In this context, the YFeO3 compound belongs to the group of antiferromagnetic orthoferrites exhibiting weak ferromagnetism (WF) and antiferromagnetism (AFM) with Néel temperature above room temperature (644 K). The compound crystallizes in orthorhombic structure with Pbnm spatial group. The WF arises from antiferromagnetic ordering via Dzyaloshinsky–Moriya interaction (DM) [9]. The system has shown high magnetic anisotropy which depends largely on the synthesis conditions. For example, the soft-chemical route is well known to have a drastic influence on magnetic properties. The size and morphology of the synthesized crystals have a notable influence in the basic magnetic features, such as residual magnetism and the coercivity (Hc) [10,11,12,13]. These facts have demonstrated that the variation of the thermal history, morphology, and particle size influence the complex interplay between weak ferromagnetism and antiferromagnetism via the small or large anisotropy fields associated to the magnetocrystalline and DM interaction. The magnetic structure of YFeO3 yields distinctive magnetic hysteresis loops originating from a small or large anisotropy field. In this compound, it is particularly interesting to know if the surface of the particles induce changes in the magnetocrystalline anisotropy, when it is functionalized by the introduction of a second phase. For this purpose, the core–shell nanocomposite looks quite promising for exploring this possibility.
Here, we have used the ALD process for attaining such a level of the interface control and thus, to fabricate the YFeO3@Al2O3 nanocomposite. By this way, uniform, conformal and homogeneous nanoscale coating of each individual magnetic particle was built. The YFeO3/Al2O3 interface fully characterized by scanning electron microscopy and transmission electron microscopy including in-column energy dispersive X-rays spectroscopy. The magnetization measurements show change the hysteresis loops profile and enhance the exchange bias effect at low magnetic field in the YFeO3. We explain the exchange anisotropy by pinning weak-ferromagnetic and antiferromagnetic domains on the surface of the particles by mean of Al2O3-shell. Our hypothesis is that the –Y–Cr–O–···–Al–O–Al– bond strength at the interface pin the motion of both domains walls during the descending and ascending magnetization in the hysteresis magnetic curves. This fact demonstrates that the WF and AFM exchange interactions are weakly coupled being this an intrinsic property on the YFeO3 compound.
2 Experimental procedure
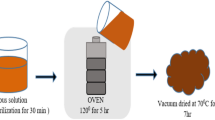
Polycrystalline powders were obtained by the molten salt method. Stoichiometric amounts of Y2O3 (99.9% Alfa-Aesar) and Fe2O3 (99.98% Sigma Aldrich) were mixed with equimolar NaCl/KCl (1:1 mol) compound which was used as a molten salt. The molar ratio of oxides/chlorides was fixed at 1:2. The precursors were mixed and homogeneously milled and, then placed in an alumina crucible and heated at a rate of 7 °C/min to reach 1100 °C. After 1 h of reaction at 1100 °C, the samples were quenched in air. The reaction products were washed in deionized water several times to eliminate salts in the samples. Then, the fine powders were dried at 120 °C for 3 h. On the other hand, Atomic layer deposition (ALD) route was used to deposit the Al2O3 coating on the YFeO3 powder particles, and thus, producing the core–shell YCrO3/Al2O3 structure. Trymethylaluminum (TMA) was used as an aluminum precursor and deionized water (H2O) as an oxidizing agent. Two half-reactions occur in the surface particles which complete one atomic layer deposition (ALD) cycle. By repeating these two self-limiting reactions, it is possible to grow layers of a few nanometers up to hundreds of nanometers. Thus, complete coatings of the YFeO3 particles were carried out with 100, 500 and 750 repetitive cycles. Details of the experimental procedure of the core–shell fabrication have been described elsewhere [15]. Phase identification of the samples was made with a Phillips X’pert-MPD diffractometer using conventional Cu Kα radiation (λ = 0.15418 nm). Data were collected in the range of 20° < 2θ < 80° with 0.02° step scan and 1 s. The microstructural characterization of the powders was performed using scanning electron microscopy (JEOL JSM-5300) and transmission electron microscopy using a scanning transmission electron microscope (FEI Tecnai G2 F20 S-TWIN operating at 200 kV). Average particle size was calculated for particles on the micrograph by measuring about 100–200 particles and using the ImageJ software. High angle annular dark field (HAADF) was performed in the same TEM along with in-column energy dispersive X-rays spectroscopy analysis. Magnetization was measured with PPMS (Quantum Design) magnetometer. The magnetization vs applied magnetic field (Happl) measurements were performed at 5 and 300 K with ± 3 T.
3 Results and discussion
Figure 1 shows the experimental X-ray diffraction (XRD) patterns for the YFeO3 sample. The phases obtained are assigned to YFeO3 with orthorhombic structure and space group Pbnm. For clarity, the YFeO3 reference pattern (bars) is showed (ICDD No. 23011) at the bottom of Fig. 1. All peaks are indexed without the presence of spurious second phases. Figure 2a, b shows the SEM images of the synthesized powders sample and the particle size distribution. It was observed that after 1 h of molten-salt reaction, the grains grew equiaxially forming rounded crystals with a size distribution of less than ~ 1-micron average as can be seen in the graph of Fig. 2a, b. Once the particle size was fully characterized, each particle was covered with aluminum oxide layers to produce the core–shell assemble. The YFeO3@Al2O3 structure was observed in samples coated by 100, 500 and 750 ALD-cycles using TEM images as can be seen in Fig. 3a–c. There, the Al2O3-shell interface (gray interface) around the YFeO3 core (dark zone) can be clearly seen. The images show the YFeO3 particles coated with different thickness of amorphous Al2O3. Using the representative TEM images shown in Fig. 3, the shell thickness was directly measured from the particle appearing in each image. Uniform, conformal and homogenous Al2O3-coating presented thickness from ~ 14 nm for 100 ALD-cycles, ~ 76 nm for 500 ALD-cycles to ~ 113 nm for 750 ALD-cycle. The growth rate of 0.15 nm per ALD-cycle is in agreement with that reported in previous works [14,15,16]. Furthermore, the HAADF-STEM image presents the brightest zone at the core consistently with the higher atomic number expected for this material (presumably YFeO3) when compared to the shell material (presumably Al2O3). On the same HAADF-STEM image a yellow line is illustrated indicating the trajectory followed by the electron beam along the sample to perform an EDXS line-scan analysis to identify the elemental composition of the shell and core. Figure 3e shows the intensity profiles from the performed EDXS line-scan corresponding to Fe, Y, O and Al. It can be seen that the Fe and Y signal follow a similar profile starting to increase their signal from almost zero at similar locations (20 nm from the line-scan) due to the expected presence of YFeO3 at the core. While the Al profile presents considerable signal from the beginning of the line-scan, corresponding to the shell location as it can be seen in Fig. 3d image (see the yellow line). Therefore, the presence of the Al2O3 shell coating the YFeO3 can be confirmed. This elemental analysis shows that the particles are completely covered with Al2O3 confirming the expected Y@Al core–shell array. It is worth noting that the ALD process allows a uniform and conformal covering with Al2O3 of the sharp corners and rounded edges of each particle as seen in Fig. 3a. Microstructure and XRD studies (not shown) do not show a signal of the shell crystallization. The amorphous lattice of the Al2O3-shell is a common characteristic of the ALD-process [17, 18].
TEM images and energy dispersive X-ray (EDX) line-scan profiles of the YFeO3/Al2O3. a–c TEM images for 100, 500 and 750 ALD-cycles. d–e HAADF-STEM image for 100 ALD-cycles sample along with EDX line scan-profiles corresponding to Al, Y, Fe and O signal from the line-scan performed following the yellow bar direction showed in image d
We have recently reported that the nanometric shell features have a notable effect on electric transport and magnetic properties [6, 16]. Here, interesting effects can be observed in the YFeO3/Al2O3 core–shell arrangement. Figure 4a, b show the magnetic M-H curves at 10 and 300 K for pristine and coated samples. The pristine YFeO3 sample shows an unsaturated magnetization with constricted M(H) loops characterized by a low coercive field at 10 and 300 K, which indicates that the YFeO3 is behaving as a magnetically soft orthoferrite compound. However, the YFeO3 ceramic compound has shown to behave as soft and hard magnet depending on crystal size and morphology. Recently, S. Madolapa et al. and others [12, 13, 19] found very similar M(H) profile hysteresis curves to that reported in this work assuming a large magnetic anisotropy as a consequence of crystal size. Recently, V. I. Povkov et al. [20] found a strong dependence of the M-H curves with the crystal morphology. For example, they found that the soft magnetic feature is present in crystals of nanometric size with isometric form, whereas rod-shape and plate-like-shape samples showed hard magnetic feature. These results indicate that the magnetocrystalline anisotropy in YFeO3 plays a crucial role because the easy axis of magnetization is strongly coupled with the crystal shape. Here, equiaxial or rounded crystal shape (see Fig. 3) decreases the magnetic anisotropy since the net spins rotate in direction of the magnetic field increasing rapidly the magnetization in a low applied magnetic field, Happl in bulk. This fact produces a low coercive field, Hc (soft magnetic behavior) in YFeO3. Subsequently, the nonsaturated magnetic hysteresis loops in the pristine sample indicates antiferromagnetic order with a slight canting of Fe spins giving rise to a weak ferromagnetic moment (WFM) that essentially dominates from 10 to 300 K as is seen in graph 1 and 5 of Fig. 4a, b. The remanence (Mr) slightly decreases from 0.75 emu/g at T = 10 K to 0.55 emu/g for T = 300 K in YFeO3. These results are in agreement with those reported by Wang et al. [12] and S. Mathur et al. [13] on high-quality YFeO3 nano-crystalline samples. Notorious changes in the hysteresis loops are seen for the coated samples. The hysteresis loops profile does not show significant changes between 10 and 300 K for pristine and coated samples being the hysteresis loops more robust at 10 K. Two remarkable effects are observed in the M-H curves in Fig. 4a, b. (a) Magnetization values obtained at 30 kOe decrease as the Al2O3-shell thickness increases and (b) the M-H curves also show a shift of the hysteresis loops towards the negative applied field axis as seen in the inset of Fig. 4a, b. About the first effect, we infer that the magnetization decreases by the non-magnetic contribution of the trimethylaluminium and water molecules. A plausible explanation is that the diamagnetic contribution of the amorphous shell containing Al and oxygen increases as the thickness of the shell increases. This contribution, we assume, decreases the magnetization at 30 kOe from 1.17 to 67 emu/g at 10 K and 0.94 to 0.50 emu/g at 300 K (see Table 1). That is, as the thickness of the coating grows, the diamagnetic contribution also increases, reducing the total magnetization at high-applied magnetic fields. The reduction of the magnetic features (Hc, Mr) by the increase of the Al2O3-shell thickness has also been observed in magnetic nanoparticles of FePt/Al2O3 composite thin films [21]. About the second effect; for the pristine sample, we observe an asymmetry in the M-H curve, which is small at 10 and 300 K, but this asymmetry is increased towards negative values for the Al2O3-shell samples as is seen in the inset of Fig. 4a, b. This peculiar behavior is also-called exchange bias or exchange anisotropy (EB). It is well known that the exchange-bias effects arise when the FM and AFM domains are coupled through an interface [22]. In general, the EB effect has been observed in some orthoferrites and orthochromites, particularly in doped samples, when the FM and AFM domains are constricted by the crystal-shape and the nanometric size [23,24,25]. In particular, YFeO3 has an antiferromagnetic ordering below of TN ~ 640 K with a magnetic configuration of Γ4(Gx, Ay, Fz) following the Bertaut notation [26]. In other words, the antiferromagnetism arises from the spin-arrangement of the Fe+3 ions along the a-axis (Gx) and, at the same time, they develop weak ferromagnetism with spin-arrangement along the c-axis (Fz). In such spin configuration, the AFM and weak-ferromagnetic domains coexist and they can induce exchange anisotropy depending on crystal anisotropy. Recently, R. Maiti et al. 11 found that the exchange bias effect is energetically favorable in monocrystalline YFeO3 compound. The result shown here suggests that both WFM and AFM domains in close contact lead to an additional anisotropy via exchange coupling on the YFeO3@Al2O3 particles (see inset in Fig. 4a, b). For clarity, the exchange-bias field, HEB and exchange–bias remanent magnetization, MEB values at 10 and 300 K are listed in Table 1 for pristine and coated samples. It is noted that the HEB increases for 500 cycles without significant increase of the HEB for 750 cycles at 300 K. At 10 K, the magnitude of HEB decreases having an even lower value for 750 cycles. A question that arises immediately is what is the origin of such exchange anisotropy? We infer that the EB enhancement is an effect occurring at the interface between YFeO3 particles and the Al2O3-Shell. We must remember that the shell of Al2O3 is non-magnetic and therefore should not contribute to magnetism. For pristine YFeO3, the negative exchange bias is associated with the WF and AFM domains weakly coupled, HEB ~ − 40 Oe. Once the core–shell structure is formed, there is a large contact surface area and likely a strong bond between YFeO3 and Al2O3 at the interface. This fact pins the motion of the WFM domains walls weakly coupled with AFM domains at the surface of the particles. i.e., at Happl = 0, during descending magnetization branch, the weak ferromagnetic domains produce a finite coercive field, Hc. When the Happ are reversed, the ascending magnetization branches at Happl = 0 is asymmetric with the descending branch due to the motion of weak ferromagnetic domains walls who are pinned by the bond Al–O–Al–···–Cr–Y–O– on the interface prevailing the motion of the AFM domains. In addition, the values obtained of the HEB (Table 1) also infer that this HEB is not dependent on the Al2O3-shell thickness. When the Al2O3-shell-thickness increases from 76 to 113 nm the HEB increases only ~ 500 Oe at 300 K and the HEB values decrease at 10 K. The explanation that we propose here is, once the particles are coated with Al2O3-shell, the motion of the WFM and AFM domains walls will depend on the morphology, roughness, cluster characteristic, superficial defects (grain boundaries) and other imperfections confined in the interface of the coated particles. Thus, the results make evident that the ferromagnetic and antiferromagnetic domains are energetically favorable and they are weakly coupled (exchange–bias effect) in the YFeO3 ceramic compound. The results showed here demonstrate that the variation of the morphology, crystallite size and the interface can reveal new phenomena having a strong influence on the complex interplay between weak ferromagnetism and antiferromagnetic interaction via the small or large anisotropy fields associated to the magnetocrystalline and DM interaction.
4 Conclusion
Here, we have studied the magnetic properties of the YFeO3@Al2O3 core–shell assemble, grown by the atomic layer deposition method following the procedure reported before [16]. Using a growth rate of 0.15 nm per cycle was possible to obtain uniform and well-controlled shell thickness of Al2O3 around YFeO3 particles. The resulting particles were coated with amorphous alumina with ~ 14, ~ 76 and ~ 114 nm thickness. We found that the magnetization values decreases and the M-H profile slightly changes with increasing shell-thickness. This fact is due to the diamagnetic contribution of the Al2O3-shell. Furthermore, negative exchange-bias is observed at low magnetic field. We propose that the enhancement of the exchange bias is due to the pinning of the weak ferromagnetic domains to the YFeO3/Al2O3 interface inhibiting domains walls motion. The no dependence of the EB field with the Al2O3-shell thickness confirms that the antiferromagnetic domains are pinned to YFeO3/Al2O3 interface.
References
Caruso F (2001) Nanoengineering of particle surfaces. Adv Mater 13(1):11–22
Ghosh Chaudhuri R, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis mechanisms, characterization, and applications. Chem Rev 112(4):2373–2433
Zhang S, Sun D, Fu Y, Du H (2003) Recent advances of superhard nanocomposite coatings: a review. Surf Coat Technol 167(2–3):113–119
Tiznado H, Dominguez D, de la Cruz W, Machorro R, Curiel M, Soto G (2012) TiO2 and Al2O3 ultra thin nanolaminates growth by ALD; instrument automation and films characterization. Rev Mex Fis 58:459–465
Tiznado H, Domínguez D, Muñoz-Muñoz F, Romo-Herrera J, Machorro R, Contreras OE, Soto G (2014) Pulsed-bed atomic layer deposition setup for powder coating. Powder Technol 267:201–207
Durán A, Tiznado H, Romo-Herrera JM, Domínguez D, Escudero R, Siqueiros JM (2014) Nanocomposite YCrO3/Al2O3: characterization of the core–shell, magnetic properties, and enhancement of dielectric properties. Inorg Chem 53(10):4872–4880
Dominguez D, Tiznado H, Borbon-Nuñez HA, Muñoz-Muñoz F, Romo-Herrera JM, Soto G (2016) Enhancing the oxidation resistance of diamond powder by the application of Al2O3 conformal coat by atomic layer deposition. Diam Relat Mater 69:108–113
Mazaleyrat F, Varga L (2000) Ferromagnetic nanocomposites. J Magn Magn Mater 215–216:253–259
Treves D (1962) Magnetic studies of some orthoferrites. Phys Rev 125(6):1843–1853
Zhang W, Fang C, Yin W, Zeng Y (2013) One-step synthesis of yttrium orthoferrite nanocrystals via sol–gel auto-combustion and their structural and magnetic characteristics. Mater Chem Phys 137(3):877–883
Maiti R, Basu S, Chakravorty D (2009) Synthesis of nanocrystalline YFeO3 and its magnetic properties. J Magn Magn Mater 321(19):3274–3277
Wang M, Wang T, Song S-H, Ravi M, Liu R-C, Ji S-S (2017) Effect of calcination temperature on structural, magnetic and optical properties of multiferroic YFeO3 nanopowders synthesized by a low temperature solid-state reaction. Ceram Int 43(13):10270–10276
Mathur S, Veith M, Rapalaviciute R, Shen H, Goya GF, Martins Filho WL, Berquo TS (2004) Molecule derived synthesis of nanocrystalline YFeO3 and investigations on its weak ferromagnetic behavior. Chem Mater 16(10):1906–1913
Valdesueiro D, Meesters G, Kreutzer M, van Ommen J (2015) Gas-phase deposition of ultrathin aluminium oxide films on nanoparticles at ambient conditions. Materials 8(3):1249–1263
Rammula R, Aarik L, Kasikov A, Kozlova J, Kahro T, Matisen L, Niilisk A, Alles H et al (2013) Atomic layer deposition of aluminum oxide films on graphene. IOP Conf Ser Mater Sci Eng 49:012014
Durán A, Moxca L, Tiznado H, Romo-Herrera JM, Herrera M, Siqueiros JM (2016) YCrO3/Al2O3 core–shell design: the effect of the nanometric Al2O3-shell on dielectric properties. J Am Ceram Soc 99(10):3382–3388
Jakschik S, Schroeder U, Hecht T, Gutsche M, Seidl H, Bartha JW (2003) Crystallization behavior of thin ALD-Al2O3 films. Thin Solid Films 425(1–2):216–220
Barbos C, Blanc-Pelissier D, Fave A, Blanquet E, Crisci A, Fourmond E, Albertini D, Sabac A et al (2015) Characterization of Al2O3 thin films prepared by thermal ALD. Energy Proc 77:558–564
Madolappa S, Ponraj B, Bhimireddi R, Varma KBR (2017) Enhanced magnetic and dielectric properties of Ti-doped YFeO3 ceramics. J Am Ceram Soc 100(6):2641–2650
Popkov VI, Almjasheva OV, Semenova AS, Kellerman DG, Nevedomskiy VN, Gusarov VV (2017) Magnetic properties of YFeO3 nanocrystals obtained by different soft-chemical methods. J Mater Sci Mater Electron 28(10):7163–7170
Kong J-Z, Gong Y-P, Li X-F, Li A-D, Zhang J-L, Yan Q-Y, Wu D (2011) Magnetic properties of FePt nanoparticle assemblies embedded in atomic-layer-deposited Al2O3. J Mater Chem 21(13):5046
Nogués J, Schuller IK (1999) Exchange bias. J Magn Magn Mater 192(2):203–232
Ahmadvand H, Salamati H, Kameli P, Poddar A, Acet M, Zakeri K (2010) Exchange bias in LaFeO3 nanoparticles. J Phys D Appl Phys 43(24):245002
Jaiswal A, Das R, Adyanthaya S, Poddar P (2011) Surface effects on morin transition, exchange bias, and enchanced spin reorientation in chemically synthesized DyFeO3 nanoparticles. J Phys Chem C 115(7):2954–2960
Durán A, Escamilla R, Escudero R, Morales F, Verdín E (2018) Reversal magnetization, spin reorientation, and exchange bias in YCrO3 doped with praseodymium. Phys Rev Mater 2(1):014409
Bertaut EF (1968) Representation analysis of magnetic structures. Acta Crystallogr Sect A Cryst Phys Diffr Theor Gen Crystallogr 24(1):217–231
Acknowledgements
A.D. and J. S. thank PAPIIT-UNAM projects IN101919 and IN105317. J. M. R. –H acknowledges UNAM for DGAPA-PAPIIT project IN105719 and CONACyT for Fordecyt project 272894. H. Tiznado thank to DGAPA PAPIIT projects IN110018 e IN112117. J. L. Moxca thanks CONACyT for the scholarship and UdeA, Colombia for the facilities provided. We thank F. Ruiz, I. Gradilla, E. Aparicio, J. Mendoza and D. Dominguez for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
H. A. Borbón-Nuñez belongs to Catedras-CONACYT program.
Rights and permissions
About this article
Cite this article
Durán, A., Moxca, L., Borbón–Núñez, H.A. et al. The role of the interface on magnetic properties for YFeO3@Al2O3 core–shell structure. SN Appl. Sci. 1, 1331 (2019). https://doi.org/10.1007/s42452-019-1341-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-1341-3