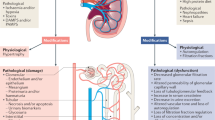
Abstract
There has been considerable progress over the last decade in the standardization of the acute kidney injury (AKI) definition with the publication of the RIFLE, AKIN, KDIGO and ERBP classification criteria. However, these classification criteria still rely on imperfect parameters such as serum creatinine and urinary output. The use of timed urine collections, kinetic eGFR (estimated glomerular filtration rate), real time measurement of GFR and direct measures of tubular damage can theoretically aid in a more timely diagnosis of AKI and improve patients’ outcome. There has been an extensive search for new biomarkers indicative of structural tubular damage but it remains controversial whether these new markers should be included in the current classification criteria. The use of these markers has also led to the creation of a new concept called subclinical AKI, a condition where there is an increase in biomarkers but without clinical AKI, defined as an increase in serum creatinine and/or a decrease in urinary output. In this review we provide a framework on how to critical appraise biomarker research and on how to position the concept of subclinical AKI. The evaluation of biomarker performance and the usefulness of the concept ‘subclinical AKI’ requires careful consideration of the context these biomarkers are used in (clinical versus research setting) and the goal we want to achieve (risk assessment versus prediction versus early diagnosis versus prognostication). It remains currently unknown whether an increase in biomarkers levels without functional repercussion is clinically relevant and whether including biomarkers in classification criteria will improve patients’ outcome.
Similar content being viewed by others
References
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8(4):R204–R212
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG et al (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2):1–8
Kidney disease (2012) Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2:1–138
Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L et al (2012) A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 27(12):4263–4272
Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C et al (2015) The definition of acute kidney injury and its use in practice. Kidney Int 87(1):62–73
Vanmassenhove J, Glorieux G, Hoste E, Dhondt A, Vanholder R, Van BW (2013) Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care 17(5):1–10
Quan S, Pannu N, Wilson T, Ball C, Tan Z, Tonelli M et al (2016) Prognostic implications of adding urine output to serum creatinine measurements for staging of acute kidney injury after major surgery: a cohort study. Nephrol Dial Transplant 31(12):2049–2056
Endre ZH, Pickering JW, Walker RJ (2011) Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI). Am J Physiol Renal Physiol 301(4):F697–F707
Waikar SS, Bonventre JV (2009) Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20(3):672–679
Chen S (2013) Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J Am Soc Nephrol 24(6):877–888
Pickering JW, Frampton CM, Walker RJ, Shaw GM, Endre ZH (2012) Four hour creatinine clearance is better than plasma creatinine for monitoring renal function in critically ill patients. Crit Care (London England) 16(3):R107
Haase M, Kellum JA, Ronco C (2012) Subclinical AKI—an emerging syndrome with important consequences. Nat Rev Nephrol 8(12):735–739
Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G et al (2011) The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 57(17):1752–1761
Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA et al (2014) Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 85(3):513–521
Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M et al (2012) Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol 59(3):246–255
Di Somma S, Magrini L, De Berardinis B, Marino R, Ferri E, Moscatelli P et al (2013) Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care (London England) 17(1):R29
Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM et al (2014) Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol 25(5):1063–1071
Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR (2012) Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol 7(8):1224–1233
Anaya-Ayala JE, Ismail N, Reardon MJ, Peden EK (2012) Endovascular salvage of a right brachial artery-right atrium hemodialysis graft using a covered endoprosthesis. J Vasc Access 13(4):520–523
Albert C, Albert A, Kube J, Bellomo R, Wettersten N, Kuppe H et al (2018) Urinary biomarkers may provide prognostic information for subclinical acute kidney injury after cardiac surgery. J Thorac Cardiovasc Surg 155(6):2441–2452 e13
McWilliam SJ, Antoine DJ, Jorgensen AL, Smyth RL, Pirmohamed M (2018) Urinary biomarkers of aminoglycoside-induced nephrotoxicity in cystic fibrosis: kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin. Science 8(1):5094
Nehus E, Kaddourah A, Bennett M, Pyles O, Devarajan P (2017) Subclinical kidney injury in children receiving nonsteroidal anti-inflammatory drugs after cardiac surgery. J Pediatr 189:175–180
Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S (2006) Neutrophil-gelatinase-associated lipocalin and renal function after percutaneous coronary interventions. Am J Nephrol 26(3):287–292
Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Dobrzycki S (2007) Neutrophil gelatinase-associated lipocalin (NGAL) correlations with cystatin C, serum creatinine and eGFR in patients with normal serum creatinine undergoing coronary angiography. Nephrol Dial Transplant 22(1):295–296
Kane-Gill SL, Smithburger PL, Kashani K, Kellum JA, Frazee E (2017) Clinical relevance and predictive value of damage biomarkers of drug-induced kidney injury. Drug Saf 40(11):1049–1074
Bellomo R, Bagshaw S, Langenberg C, Ronco C (2007) Pre-renal azotemia: a flawed paradigm in critically ill septic patients? Contrib Nephrol 156:1–9
Parikh CR, Coca SG (2010) Acute kidney injury: defining prerenal azotemia in clinical practice and research. Nat Rev Nephrol 6(11):641–642
Belcher JM, Parikh CR (2011) Is it time to evolve past the prerenal azotemia versus acute tubular necrosis classification? Clin J Am Soc Nephrol 6(10):2332–2334
Schneider AG, Bellomo R (2013) Urinalysis and pre-renal acute kidney injury: time to move on. Crit Care (London, England) 17(3):141
Au V, Feit J, Barasch J, Sladen RN, Wagener G (2016) Urinary neutrophil gelatinase-associated lipocalin (NGAL) distinguishes sustained from transient acute kidney injury after general surgery. Kidney Int Rep 1(1):3–9
Xu K, Rosenstiel P, Paragas N, Hinze C, Gao X, Huai Shen T et al (2017) Unique transcriptional programs identify subtypes of AKI. J Am Soc Nephrol 28(6):1729–1740
Devarajan P (2017) Acute kidney injury: acute kidney injury: still misunderstood and misdiagnosed. Nat Rev Nephrol 13(3):137–138
Molitoris BA. Rethinking CKD, Evaluation (2017) Should we be quantifying basal or stimulated GFR to maximize precision and sensitivity? Am J Kidney Dis 69(5):675–683
Molitoris BA, Reilly ES (2016) Quantifying glomerular filtration rates in acute kidney injury: a requirement for translational success. Semin Nephrol 36(1):31–41
Bosch JP, Lew S, Glabman S, Lauer A (1986) Renal hemodynamic changes in humans. Response to protein loading in normal and diseased kidneys. Am J Med 81(5):809–815
Claus BO, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ (2013) Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care 28(5):695–700
Udy AA, Baptista JP, Lim NL, Joynt GM, Jarrett P, Wockner L et al (2014) Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med 42(3):520–527
Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA et al (2011) A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care (London England) 15(3):R139
Husain-Syed F, Ferrari F, Sharma A, Danesi TH, Bezerra P, Lopez-Giacoman S et al (2018) Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg 105(4):1094–1101
Vanmassenhove J, Vanholder R, Nagler E, Van BW (2013) Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant 28(2):254–273
Lameire NH, Vanholder RC, Van Biesen WA (2011) How to use biomarkers efficiently in acute kidney injury. Kidney Int 79(10):1047–1050
Malhotra R, Siew ED (2017) Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol 12(1):149–173
Vanmassenhove J, Glorieux G, Lameire N, Hoste E, Dhondt A, Vanholder R et al (2015) Influence of severity of illness on neutrophil gelatinase-associated lipocalin performance as a marker of acute kidney injury: a prospective cohort study of patients with sepsis. BMC Nephrol 16:18
Giasson J, Li GH, Chen Y (2011) Neutrophil gelatinase-associated lipocalin (NGAL) as a new biomarker for non-acute kidney injury (AKI) diseases. Inflamm Allergy Drug Targets 10(4):272–282
Decavele AS, Dhondt L, De Buyzere ML, Delanghe JR (2011) Increased urinary neutrophil gelatinase associated lipocalin in urinary tract infections and leukocyturia. Clin Chem Lab Med 49(6):999–1003
Martensson J, Bellomo R (2014) The rise and fall of NGAL in acute kidney injury. Blood Purif 37(4):304–310
Langenberg C, Bagshaw SM, May CN, Bellomo R (2008) The histopathology of septic acute kidney injury: a systematic review. Crit Care 12(2):R38
Moledina DG, Hall IE, Thiessen-Philbrook H, Reese PP, Weng FL, Schroppel B et al (2017) Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am J Kidney Dis 70(6):807–816
Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M et al (2013) Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care 17(1):R25
Ronco C (2016) Acute kidney injury: from clinical to molecular diagnosis. Crit Care (London England) 20(1):201
Ronco C (2016) Cell-cycle arrest biomarkers: the light at the end of the acute kidney injury tunnel. Nephrol Dial Transplant 31(1):3–5
Johnson ACM, Zager RA (2018) Mechanisms underlying increased TIMP2 and IGFBP7 urinary excretion in experimental AKI. J Am Soc Nephrol 29(8):2157–2167
Liu C, Lu X, Mao Z, Kang H, Liu H, Pan L et al (2017) The diagnostic accuracy of urinary [TIMP-2]·[IGFBP7] for acute kidney injury in adults: a PRISMA-compliant meta-analysis. Medicine (Baltimore) 96(27):e7484
Bell M, Larsson A, Venge P, Bellomo R, Martensson J (2015) Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Mark 2015:158658
Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M et al (2016) Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis 68(1):19–28
Lameire N, Vanmassenhove J, Van Biesen W, Vanholder R (2016) The cell cycle biomarkers: promising research, but do not oversell them. Clin Kidney J 9(3):353–358
Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J et al (2017) Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med 43(11):1551–1561
Gocze I, Jauch D, Gotz M, Kennedy P, Jung B, Zeman F et al (2018) Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg 267(6):1013–1020
Singh AN, Kilambi R (2018) Biomarker-guided intervention to prevent AKI or KDIGO care bundle to prevent AKI in high-risk patients undergoing major surgery? Ann Surg 268(6):e67–e68
Pickering JW, Endre ZH (2012) Challenges facing early detection of acute kidney injury in the critically ill. World J Crit Care Med 1(3):61–66
Chen S (2018) Kinetic glomerular filtration rate in routine clinical practice-applications and possibilities. Adv Chronic Kidney Dis 25(1):105–14
Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR et al (2010) Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 77:536–542
Pianta TJ, Endre ZH, Pickering JW, Buckley NA, Peake PW (2015) Kinetic estimation of GFR improves prediction of dialysis and recovery after kidney transplantation. PLoS One 10(5):e0125669
Seelhammer TG, Maile MD, Heung M, Haft JW, Jewell ES, Engoren M (2016) Kinetic estimated glomerular filtration rate and acute kidney injury in cardiac surgery patients. J Crit Care 31(1):249–254
Dewitte A, Joannes-Boyau O, Sidobre C, Fleureau C, Bats ML, Derache P et al (2015) Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol 10(11):1900–1910
O’Sullivan ED, Doyle A (2017) The clinical utility of kinetic glomerular filtration rate. Clin Kidney J 10(2):202–208
Wang E, Meier DJ, Sandoval RM, Von Hendy-Willson VE, Pressler BM, Bunch RM et al (2012) A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int 81(1):112–117
Rizk DV, Meier D, Sandoval RM, Chacana T, Reilly ES, Seegmiller JC et al (2018) A novel method for rapid bedside measurement of GFR. J Am Soc Nephrol 29(6):1609–1613
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interests to declare.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Vanmassenhove, J., Van Biesen, W., Vanholder, R. et al. Subclinical AKI: ready for primetime in clinical practice?. J Nephrol 32, 9–16 (2019). https://doi.org/10.1007/s40620-018-00566-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-018-00566-y