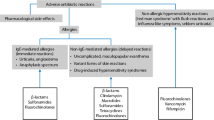
Opinion statement
Exanthemas are frequently encountered in the pediatric population and often occur while patients concomitantly receive a drug, leading to a high prevalence of suspicion of drug allergy in children. Although the vast majority of these exanthemas are due to the underlying infection, most of those patients are falsely labeled as “drug allergic” without appropriate testing, mostly due to fear of life-threatening reactions. Overdiagnosis of drug allergy constitutes a major public health problem by increasing health costs and by contributing to overall antibiotic resistance. Thus, an accurate diagnosis is considered as a major contribution to cost-effective health care and will be based on a complete allergic workup (i.e., clinical history, skin tests, in vitro tests, and/or drug provocation test). Specific aspects of the management of drug allergy have been highlighted recently, particularly regarding the importance and safety of the drug provocation test as well as the low diagnostic value of skin tests in the diagnosis of betalactam allergy.

Similar content being viewed by others
Abbreviations
- NSAID:
-
Nonsteroidal anti-inflammatory drugs
- BL:
-
Betalactams
- EBV:
-
Epstein-Barr virus
- DPT:
-
Drug provocation test
- SCARs:
-
Severe cutaneous adverse drug reactions
- AGEP:
-
Acute generalized exanthematic pustulosis
- DIHS:
-
Drug-induced hypersensitivity syndrome
- SJS:
-
Stevens-Johnson syndrome
- TEN:
-
Toxic epidermal necrolysis
- LTT:
-
Lymphocyte transformation test
- LAT:
-
Lymphocyte activation test
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rebelo Gomes E, et al. Drug allergy claims in children: from self-reporting to confirmed diagnosis. Clin Exp Allergy. 2008;38(1):191–8.
Le J, et al. Adverse drug reactions among children over a 10-year period. Pediatrics. 2006;118(2):555–62.
Lange L, Koningsbruggen SV, Rietschel E. Questionnaire-based survey of lifetime-prevalence and character of allergic drug reactions in German children. Pediatr Allergy Immunol. 2008;19(7):634–8.
Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778 e1–7.
• Caubet JC, et al. The role of penicillin in benign skin rashes in childhood: a prospective study based on drug rechallenge. J Allergy Clin Immunol. 2011;127(1):218–22. A prospective study demonstrating overdiagnosis of betalactam allergy in children and suggesting a viral cause in the majority of children developing a rash while treated by betalactam
• Ponvert C, et al. Allergy to betalactam antibiotics in children: results of a 20-year study based on clinical history, skin and challenge tests. Pediatr Allergy Immunol. 2011;22(4):411–8. One of the largest pediatric study on betalactam allergy in children, evaluating the diagnostic value of the available diagnostic tools (skin tests, specific IgE and/or drug provocation test)
Erkocoglu M, et al. Prevalence of confirmed immediate type drug hypersensitivity reactions among school children. Pediatr Allergy Immunol. 2013;24(2):160–7.
Rubio M, et al. Results of drug hypersensitivity evaluations in a large group of children and adults. Clin Exp Allergy. 2012;42(1):123–30.
Aberer W, et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: general considerations. Allergy. 2003;58(9):854–63.
• Zambonino MA, et al. Diagnostic evaluation of hypersensitivity reactions to beta-lactam antibiotics in a large population of children. Pediatr Allergy Immunol. 2014;25(1):80–7. Prospective pediatric study highlighting the importance of the drug provocation test in the diagnosis of nonimmediate benign reactions to betalactams
Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797–8.
Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264–5.
van Dijk SM, et al. The high impact of penicillin allergy registration in hospitalized patients. J Allergy Clin Immunol Pract. 2016;4(5):926–31.
•• Gomes ER, et al. Drug hypersensitivity in children: report from the pediatric task force of the EAACI Drug Allergy Interest Group. Allergy. 2016;71(2):149–61. This paper summarizes our knowledge on drug allergy in children, highlighting the lack of pediatric data, and including a general algorithm for the management of children with a suspicion of drug allergy
Schachner L, Hansen R. Pediatric Dermatology, 4th Edition, 2011.
Folster-Holst R, Kreth HW. Viral exanthems in childhood—infectious (direct) exanthems. Part 1: classic exanthems. J Dtsch Dermatol Ges. 2009;7(4):309–16.
Vega Alonso T, et al. Incidence and clinical characteristics of maculopapular exanthemas of viral aetiology. Aten Primaria. 2003;32(9):517–23.
Goodyear HM, et al. Acute infectious erythemas in children: a clinico-microbiological study. Br J Dermatol. 1991;124(5):433–8.
Folster-Holst R, Kreth HW. Viral exanthems in childhood—infectious (direct) exanthems. Part 2: other viral exanthems. J Dtsch Dermatol Ges. 2009;7(5):414–9.
• Onodi-Nagy K, et al. Amoxicillin rash in patients with infectious mononucleosis: evidence of true drug sensitization. Allergy Asthma Clin Immunol. 2015;11(1):1. This is a review discussing extensively the potential role of EBV in patients treated by amoxicillin and developing a skin rash
•• Demoly P, et al. International consensus on drug allergy. Allergy. 2014;69(4):420–37. International guidelines for the management of patients with a suspicion of drug allergy
Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007;33(1–2):124–33.
White KD, et al. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136(2):219–34. quiz 235
Atanaskovic-Markovic M, et al. Non-immediate hypersensitivity reactions to beta-lactam antibiotics in children—our 10-year experience in allergy work-up. Pediatr Allergy Immunol. 2016;27(5):533–8.
Mattheij M, de Vries E. A suspicion of antibiotic allergy in children is often incorrect. J Allergy Clin Immunol. 2012;129(2):583. author reply 583-4
Mill C, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and non-immediate reactions to amoxicillin in children. JAMA Pediatr. 2016;170(6):e160033.
Alves C, et al. Non-steroidal anti-inflammatory drug hypersensitivity in children. Allergol Immunopathol (Madr). 2017;45(1):40–7.
Guvenir H, et al. Nonsteroidal anti-inflammatory drug hypersensitivity among children. Allergy Asthma Proc. 2015;36(5):386–93.
Stur K, Karlhofer FM, Stingl G. Soluble FAS ligand: a discriminating feature between drug-induced skin eruptions and viral exanthemas. J Invest Dermatol. 2007;127(4):802–7.
Bircher AJ. Symptoms and danger signs in acute drug hypersensitivity. Toxicology. 2005;209(2):201–7.
Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–73.
Romano A, Caubet JC. Antibiotic allergies in children and adults: from clinical symptoms to skin testing diagnosis. J Allergy Clin Immunol Pract. 2014;2(1):3–12.
Blanca M, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. 2009;64(2):183–93.
Mori F, et al. Amoxicillin allergy in children: five-day drug provocation test in the diagnosis of non-immediate reactions. J Allergy Clin Immunol Pract. 2015;3(3):375–80 e1.
Moral L, et al. Short protocol for the study of paediatric patients with suspected betalactam antibiotic hypersensitivity and low risk criteria. Allergol Immunopathol (Madr). 2011;39(6):337–41.
Vezir E, et al. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol. 2016;27(1):50–4.
Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59(8):809–20.
Beeler A, et al. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy. 2008;63(2):181–8.
Caubet JC, et al. Skin tests and in vitro allergy tests have a poor diagnostic value for benign skin rashes due to beta-lactams in children. Pediatr Allergy Immunol. 2015;26(1):80–2.
•• Kuyucu S, et al. Hypersensitivity reactions to non-betalactam antibiotics in children: an extensive review. Pediatr Allergy Immunol. 2014;25(6):534–43. Exentive review of the literature summarizing the available data regarding hypersensitivity reactions to non-betalactam antibiotics in children
Ibia EO, Schwartz RH, Wiedermann BL. Antibiotic rashes in children: a survey in a private practice setting. Arch Dermatol. 2000;136(7):849–54.
Orhan F, et al. Parental-reported drug allergy in 6- to 9-yr-old urban schoolchildren. Pediatr Allergy Immunol. 2008;19(1):82–5.
Tan VA, Gerez IF, Van Bever HP. Prevalence of drug allergy in Singaporean children. Singap Med J. 2009;50(12):1158–61.
Lusini G, et al. Antibiotic prescribing in paediatric populations: a comparison between Viareggio, Italy and Funen, Denmark. Eur J Pub Health. 2009;19(4):434–8.
Blanca-Lopez N, Andreu I, Torres Jaen MJ. Hypersensitivity reactions to quinolones. Curr Opin Allergy Clin Immunol. 2011;11(4):285–91.
Burke P, Burne SR. Allergy associated with ciprofloxacin. BMJ. 2000;320(7236):679.
Iannini P, et al. Cutaneous adverse events and gemifloxacin: observations from the clinical trial program. J Chemother. 2006;18(1):3–11.
Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5(2):231–49.
Tan WC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62(2):143–6.
Chintu C, et al. Cutaneous hypersensitivity reactions due to thiacetazone in the treatment of tuberculosis in Zambian children infected with HIV-I. Arch Dis Child. 1993;68(5):665–8.
Panova LV, Ovsiankina ES. Incidence of adverse reactions to chemotherapy and their types in adolescents with tuberculosis. Probl Tuberk. 2003;1:28–30.
Nemir RL, O'Hare D. Tuberculosis in children 10 years of age and younger: three decades of experience during the chemotherapeutic era. Pediatrics. 1991;88(2):236–41.
Ormerod LP, Horsfield N. Frequency and type of reactions to antituberculosis drugs: observations in routine treatment. Tuber Lung Dis. 1996;77(1):37–42.
•• Brockow K, et al. Skin test concentrations for systemically administered drugs—an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy. 2013;68(6):702–12. Important position paper providing optimal concentration for skin tests for systemically administered drugs
Chia FL, Thong BY. Macrolide allergy: which tests are really useful? Allergol Immunopathol (Madr). 2011;39(4):191–2.
Kelly TE, Hackett PH. Acetazolamide and sulfonamide allergy: a not so simple story. High Alt Med Biol. 2010;11(4):319–23.
Eustace N, O'Hare B. Use of nonsteroidal anti-inflammatory drugs in infants. A survey of members of the Association of Paediatric Anaesthetists of Great Britain and Ireland. Paediatr Anaesth. 2007;17(5):464–9.
Neubert A, et al. The prescribing of analgesics and non-steroidal anti-inflammatory drugs in paediatric primary care in the UK, Italy and the Netherlands. Pharmacol Res. 2010;62(3):243–8.
Valkhoff VE, et al. Population-based analysis of non-steroidal anti-inflammatory drug use among children in four European countries in the SOS project: what size of data platforms and which study designs do we need to assess safety issues? BMC Pediatr. 2013;13:192.
Mayorga C, et al. Cutaneous symptoms in drug allergy: what have we learnt? Curr Opin Allergy Clin Immunol. 2009;9(5):431–6.
Kowalski ML, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy. 2013;68(10):1219–32.
•• Blanca-Lopez N, et al. Hypersensitivity reactions to nonsteroidal anti-inflammatory drugs in children and adolescents: selective reactions. J Investig Allergol Clin Immunol. 2015;25(6):385–95. Review of the literature regarding selective reactions to nonsteroidal anti-inflammatory drugs in children
Faulkner L, et al. The importance of hapten-protein complex formation in the development of drug allergy. Curr Opin Allergy Clin Immunol. 2014;14(4):293–300.
Kretz-Rommel A, Boelsterli UA. Diclofenac covalent protein binding is dependent on acyl glucuronide formation and is inversely related to P450-mediated acute cell injury in cultured rat hepatocytes. Toxicol Appl Pharmacol. 1993;120(1):155–61.
Ikegawa S, et al. The enantioselective immunoaffinity extraction of an optically active ibuprofen-modified peptide fragment. Anal Biochem. 2001;296(1):63–72.
Kowalski ML, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs (NSAIDs)—classification, diagnosis and management: review of the EAACI/ENDA(#) and GA2LEN/HANNA*. Allergy. 2011;66(7):818–29.
Hassani A, et al. Hypersensitivity to cyclooxygenase inhibitory drugs in children: a study of 164 cases. Eur J Dermatol. 2008;18(5):561–5.
Zambonino MA, et al. Drug provocation tests in the diagnosis of hypersensitivity reactions to non-steroidal anti-inflammatory drugs in children. Pediatr Allergy Immunol. 2013;24(2):151–9.
Kidon MI, et al. Hypersensitivity to paracetamol in Asian children with early onset of nonsteroidal anti-inflammatory drug allergy. Int Arch Allergy Immunol. 2007;144(1):51–6.
Yilmaz O, et al. Challenge-proven nonsteroidal anti-inflammatory drug hypersensitivity in children. Allergy. 2013;68(12):1555–61.
Ordoqui E, et al. Cross-sensitivity among oxicams in piroxicam-caused fixed drug eruption: two case reports. Allergy. 1995;50(9):741–4.
Gomez-Traseira C, et al. Paracetamol-induced fixed drug eruption at an unusual site. Recent Patents Inflamm Allergy Drug Discov. 2013;7(3):268–70.
Atanaskovic-Markovic M, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatr Allergy Immunol. 2013;24(7):645–9.
Kelso JM. Allergic reactions after immunization. Ann Allergy Asthma Immunol. 2013;110(6):397–401.
• Caubet JC, et al. Managing a child with possible allergy to vaccine. Pediatr Allergy Immunol. 2014;25(4):394–403. Review including discussion of practical aspects of the management of a child with possible allergy to vaccine
Sukumaran L, et al. Adverse events following measles, mumps, and rubella vaccine in adults reported to the Vaccine Adverse Event Reporting System (VAERS), 2003-2013. Clin Infect Dis. 2015;60(10):e58–65.
Gold M, et al. Re-vaccination of 421 children with a past history of an adverse vaccine reaction in a special immunisation service. Arch Dis Child. 2000;83(2):128–31.
Jacobs RL, Lowe RS, Lanier BQ. Adverse reactions to tetanus toxoid. JAMA. 1982;247(1):40–2.
Andre FE. Overview of a 5-year clinical experience with a yeast-derived hepatitis B vaccine. Vaccine. 1990;(8 Suppl):S74–8. discussion S79-80
McMahon BJ, et al. Frequency of adverse reactions to hepatitis B vaccine in 43,618 persons. Am J Med. 1992;92(3):254–6.
Long SS, et al. Longitudinal study of adverse reactions following diphtheria-tetanus-pertussis vaccine in infancy. Pediatrics. 1990;85(3):294–302.
Szmuness W, et al. Hepatitis B vaccine: demonstration of efficacy in a controlled clinical trial in a high-risk population in the United States. N Engl J Med. 1980;303(15):833–41.
Siegrist CA. Mechanisms underlying adverse reactions to vaccines. J Comp Pathol. 2007;137(Suppl 1):S46–50.
•• Dreskin SC, et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9(1):32. International guidelines on the management of patients with a suspicion of allergy to vaccines
Vogt T, Landthaler M, Stolz W. Generalized eczema in an 18-month-old boy due to phenoxyethanol in DPT vaccine. Contact Dermatitis. 1998;38(1):50–1.
Ghadially R, Ramsay CA. Gentamicin: systemic exposure to a contact allergen. J Am Acad Dermatol. 1988;19(2 Pt 2):428–30.
Idsoe O, et al. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38(2):159–88.
Neeno DNT. Anaphylaxis to benzathine penicillin G. Pediatr Asthma Allergy Immunol. 2000;14:329.
Romano A, et al. Diagnosis and management of drug hypersensitivity reactions. J Allergy Clin Immunol. 2011;127(3 Suppl):S67–73.
Macy E. The clinical evaluation of penicillin allergy: what is necessary, sufficient and safe given the materials currently available? Clin Exp Allergy. 2011;41(11):1498–501.
Fox S, Park MA. Penicillin skin testing in the evaluation and management of penicillin allergy. Ann Allergy Asthma Immunol. 2011;106(1):1–7.
Bousquet PJ, et al. Oral challenges are needed in the diagnosis of beta-lactam hypersensitivity. Clin Exp Allergy. 2008;38(1):185–90.
• Torres MJ, et al. Clavulanic acid can be the component in amoxicillin-clavulanic acid responsible for immediate hypersensitivity reactions. J Allergy Clin Immunol. 2010;125(2):502–505 e2. Study highlighting the possibility of specific hypersensitivity to clavulanic acid itself in patients reacting to amoxicillin-clavulanic acid
Sanchez-Morillas L, et al. Selective allergic reactions to clavulanic acid: a report of 9 cases. J Allergy Clin Immunol. 2010;126(1):177–9.
Park MA, et al. Patients with positive skin test results to penicillin should not undergo penicillin or amoxicillin challenge. J Allergy Clin Immunol. 2015;135(3):816–7.
Green GR, Rosenblum AH, Sweet LC. Evaluation of penicillin hypersensitivity: value of clinical history and skin testing with penicilloyl-polylysine and penicillin G. A cooperative prospective study of the penicillin study group of the American Academy of Allergy. J Allergy Clin Immunol. 1977;60(6):339–45.
Sogn DD, et al. Results of the National Institute of Allergy and Infectious Diseases collaborative clinical trial to test the predictive value of skin testing with major and minor penicillin derivatives in hospitalized adults. Arch Intern Med. 1992;152(5):1025–32.
Macy E, Burchette RJ. Oral antibiotic adverse reactions after penicillin skin testing: multi-year follow-up. Allergy. 2002;57(12):1151–8.
Adkinson Jr NF, et al. Routine use of penicillin skin testing on an inpatient service. N Engl J Med. 1971;285(1):22–4.
Levine BB, Zolov DM. Prediction of penicillin allergy by immunological tests. J Allergy. 1969;43(4):231–44.
Chambel M, et al. Drug provocation tests to betalactam antibiotics: experience in a paediatric setting. Allergol Immunopathol (Madr). 2010;38(6):300–6.
Waton J, et al. Negative predictive value of drug skin tests in investigating cutaneous adverse drug reactions. Br J Dermatol. 2009;160(4):786–94.
Marrs T, et al. The diagnosis and management of antibiotic allergy in children: systematic review to inform a contemporary approach. Arch Dis Child. 2015;100(6):583–8.
Ponvert C, et al. Allergy to betalactam antibiotics in children: a prospective follow-up study in retreated children after negative responses in skin and challenge tests. Allergy. 2007;62(1):42–6.
Sanz ML, Gamboa PM, Mayorga C. Basophil activation tests in the evaluation of immediate drug hypersensitivity. Curr Opin Allergy Clin Immunol. 2009;9(4):298–304.
Atanaskovic-Markovic M, et al. Immediate allergic reactions to cephalosporins and penicillins and their cross-reactivity in children. Pediatr Allergy Immunol. 2005;16(4):341–7.
Atanaskovic-Markovic M. Educational case series: beta-lactam allergy and cross-reactivity. Pediatr Allergy Immunol. 2011;22(8):770–5.
Romano A, et al. Cross-reactivity and tolerability of cephalosporins in patients with immediate hypersensitivity to penicillins. Ann Intern Med. 2004;141(1):16–22.
Novalbos A, et al. Lack of allergic cross-reactivity to cephalosporins among patients allergic to penicillins. Clin Exp Allergy. 2001;31(3):438–43.
Miranda A, et al. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J Allergy Clin Immunol. 1996;98(3):671–7.
Audicana M, et al. Allergic reactions to betalactams: studies in a group of patients allergic to penicillin and evaluation of cross-reactivity with cephalosporin. Allergy. 1994;49(2):108–13.
Sastre J, et al. Clinical cross-reactivity between amoxicillin and cephadroxil in patients allergic to amoxicillin and with good tolerance of penicillin. Allergy. 1996;51(6):383–6.
Perez Pimiento A, et al. Aztreonam and ceftazidime: evidence of in vivo cross allergenicity. Allergy. 1998;53(6):624–5.
Adkinson Jr NF. Immunogenicity and cross-allergenicity of aztreonam. Am J Med. 1990;88(3C):12S–5S. discussion 38S-42S
Saxon A, et al. Lack of cross-reactivity between aztreonam, a monobactam antibiotic, and penicillin in penicillin-allergic subjects. J Infect Dis. 1984;149(1):16–22.
Adkinson Jr NF, et al. Cross-allergenicity and immunogenicity of aztreonam. Rev Infect Dis. 1985;7(Suppl 4):S613–21.
Atanaskovic-Markovic M, et al. Tolerability of imipenem in children with IgE-mediated hypersensitivity to penicillins. J Allergy Clin Immunol. 2009;124(1):167–9.
Frumin J, Gallagher JC. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: what are the chances? Ann Pharmacother. 2009;43(2):304–15.
Romano A, et al. IgE-mediated hypersensitivity to cephalosporins: cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J Allergy Clin Immunol. 2010;126(5):994–9.
Craft JC, Siepman N. Overview of the safety profile of clarithromycin suspension in pediatric patients. Pediatr Infect Dis J. 1993;12(12 Suppl 3):S142–7.
Odio C, et al. Adverse reactions to vancomycin used as prophylaxis for CSF shunt procedures. Am J Dis Child. 1984;138(1):17–9.
Levy M, et al. Vancomycin-induced red man syndrome. Pediatrics. 1990;86(4):572–80.
Sivagnanam S, Deleu D. Red man syndrome. Crit Care. 2003;7(2):119–20.
Myers AL, et al. Defining risk factors for red man syndrome in children and adults. Pediatr Infect Dis J. 2012;31(5):464–8.
Wazny LD, Daghigh B. Desensitization protocols for vancomycin hypersensitivity. Ann Pharmacother. 2001;35(11):1458–64.
Earl HS, Sullivan TJ. Acute desensitization of a patient with cystic fibrosis allergic to both beta-lactam and aminoglycoside antibiotics. J Allergy Clin Immunol. 1987;79(3):477–83.
Spigarelli MG, Hurwitz ME, Nasr SZ. Hypersensitivity to inhaled TOBI following reaction to gentamicin. Pediatr Pulmonol. 2002;33(4):311–4.
Ben Said B, et al. Usefulness of basophil activation tests for the diagnosis of IgE-mediated allergy to quinolones. Allergy. 2010;65(4):535–6.
•• Blanca-Lopez N, et al. Hypersensitivity to nonsteroidal anti-inflammatory drugs in children and adolescents: cross-intolerance reactions. J Investig Allergol Clin Immunol. 2015;25(4):259–69. Review of the literature regarding cross intolerance reactions to nonsteroidal anti-inflammatory drugs in children
Asero R. Intolerance to nonsteroidal anti-inflammatory drugs might precede by years the onset of chronic urticaria. J Allergy Clin Immunol. 2003;111(5):1095–8.
Church MK, et al. Chronic spontaneous urticaria in children: itching for insight. Pediatr Allergy Immunol. 2011;22(1 Pt 1):1–8.
Kidon MI, et al. Early presentation with angioedema and urticaria in cross-reactive hypersensitivity to nonsteroidal antiinflammatory drugs among young, Asian, atopic children. Pediatrics. 2005;116(5):e675–80.
Olze H, Lau S, Forster U. Samter’s triad and eicosanoid imbalance in children with recurrent nasal polyps. Pediatr Allergy Immunol. 2012;23(5):500.
Reisfeld S, Goldberg A, Confino-Cohen R. Management of patients with known drug hypersensitivity in an emergency department in Israel. Int Arch Allergy Immunol. 2011;155(4):361–6.
Viola M, et al. Assessing potential determinants of positive provocation tests in subjects with NSAID hypersensitivity. Clin Exp Allergy. 2011;41(1):96–103.
Blanca-Lopez N, et al. Value of the clinical history in the diagnosis of urticaria/angioedema induced by NSAIDs with cross-intolerance. Clin Exp Allergy. 2013;43(1):85–91.
Dona I, et al. Characteristics of subjects experiencing hypersensitivity to non-steroidal anti-inflammatory drugs: patterns of response. Clin Exp Allergy. 2011;41(1):86–95.
Zent O, et al. Immediate allergic reactions after vaccinations—a post-marketing surveillance review. Eur J Pediatr. 2002;161(1):21–5.
Bohlke K, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112(4):815–20.
Wood RA, et al. An algorithm for treatment of patients with hypersensitivity reactions after vaccines. Pediatrics. 2008;122(3):e771–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Jean-Christoph Caubet declares that he has no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Drug Allergy
Rights and permissions
About this article
Cite this article
Caubet, JC. How to Manage Drug-Induced Exanthema in Children. Curr Treat Options Allergy 4, 222–238 (2017). https://doi.org/10.1007/s40521-017-0131-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-017-0131-7