Abstract
Objective
This prospective, multicenter, non-interventional cohort study enrolling human immunodeficiency virus (HIV)-1-infected, virally suppressed adult outpatients in Italy aimed to describe results obtained from patient-reported outcome questionnaires regarding treatment satisfaction and symptom perceptions in HIV-1-positive patients who switched to cobicistat-boosted darunavir antiretroviral regimens, coming from ritonavir-boosted protease inhibitors.
Methods
Patients entered this study between June 2016 and February 2017, once their treating physician had considered them eligible for cobicistat-boosted darunavir-based treatment as per clinical practice. Patients’ satisfaction regarding regimen and current symptom burdens were assessed using two previously validated, patient-reported outcome questionnaires: HIV Treatment Satisfaction Questionnaire (HIV-TSQ) and HIV Symptoms Distress Module (HIV-SDM). These questionnaires were administered at prespecified time-points: enrollment (Visit 1), 4–8 weeks later (Visit 2), and 48 ± 6 weeks after study enrollment (Visit 4). Data of patient-reported outcome total scores for both questionnaires are presented as median with 25th–75th percentiles. Questionnaires scores were analyzed overall and stratified by gender when applicable. A p value of less than 0.05 was considered statistically significant. A sensitivity analysis was conducted to evaluate the role of lost to follow-up, using the “last observation carried forward” method.
Results
A total of 348 patients were enrolled in this study; 296 patients (208 male and 88 female) provided both evaluable HIV-TSQ and HIV-SDM at enrollment and at 4–8 weeks, while 250 patients (174 male and 76 female) provided questionnaire data at enrollment and at 48 ± 6 weeks. The total scores of HIV-TSQ showed improvements in patient satisfaction in the overall population both at Visit 2 and Visit 4 (p < 0.001, sign test) and also when stratified by gender throughout the study period. In addition, the overall burden of symptoms, as shown by the HIV-SDM scores, decreased.
Conclusions
Switching to a cobicistat-boosted darunavir-based therapy led to overall increased patient satisfaction and reduced symptom burden when compared with previous regimens. The use of patient-reported outcomes in clinical daily practice could provide a useful tool towards achieving guideline goals to achieve “fourth 90”, having 90% of virally suppressed patients with a good health-related quality of life.
Similar content being viewed by others
Cobicistat-boosted darunavir might represent a valuable switching option for patients with stable human immunodeficiency virus currently in therapy with a protease inhibitor with ritonavir. |
Patient-reported outcomes could be useful tools for capturing patient satisfaction and treatment tolerability, in view of achieving the “fourth 90” in human immunodeficiency virus, namely a good quality of life. The use of such tools in observational studies could help better understand patients’ needs. |
1 Introduction
Currently available antiretroviral therapies (ARTs) have transformed human immunodeficiency virus (HIV) infections from a previously fatal disease to one that is more chronic in nature; indeed, patients with HIV currently have life expectancies that are nearly comparable to HIV-uninfected individuals [1]. However, in the absence of an HIV cure, patients still require lifelong therapy, effective in maintaining viral suppression if taken according to prescription [2, 3]. The Joint United Nations Programme on HIV and AIDS established 90-90-90 testing and treatment targets in 2014 with the ambitious goal of ending the global HIV epidemic; these guidelines were later endorsed by the World Health Organization in the Global Health Sector Strategy on HIV 2016–2021. More recently, these proposed metrics were updated to include a fourth target, which was to ensure that 90% of virally suppressed patients have good health-related quality of life [4, 5]. Research efforts have thus been directed towards regimen simplification, as demonstrated by the success of fixed-dose combinations and single-tablet regimens [6, 7], as well as towards the use of validated instruments or questionnaires to better evaluate perspectives of patients who are HIV positive with regard to ARTs [8,9,10].
The convenience achieved through switching from two separate tablets of a protease inhibitor with ritonavir (PI/r) to a fixed-dose single tablet of both darunavir and cobicistat (DRV/c) [Rezolsta®] [11], as well as switching from ritonavir to the new, safer pharmacokinetic booster cobicistat [12], fall within the scope of answering patient needs of treatment simplification and increased tolerability.
Among the available protease inhibitors (PIs), DRV is increasingly favored because of its high genetic resistance barrier, increased tolerability, and its availability as the only PI-containing single-tablet regimen [13,14,15,16,17]. Cobicistat lacks antiviral activity and, as a booster, has been shown to be as effective as ritonavir [18]. Moreover, cobicistat is less prone to drug–drug interactions owing to its more selective inhibition of cytochrome P450 isoenzymes. However, real-world patient satisfaction when switching to a single-tablet DRV/c combination remained to be assessed.
Presently, questionnaires used to assess patient-reported outcomes (PROs) are mainly used in HIV registrational trials to evaluate the effects of a new treatment on several factors, including health-related quality-of-life symptoms, physical and cognitive function, as well as disability and severity of disease as shown by the use of the HIV-Treatment Satisfaction Questionnaire (HIV-TSQ) in recent trials [19,20,21,22]. With a focus on patient-centered healthcare systems, PRO measures are progressively taken into account in routine clinical care as they are considered valuable instruments to integrate clinical and laboratory data with patient perspectives [23, 24].
This study described the use of PROs in the STart Of REzolsta (ST.O.RE.) study (NCT02926456), a prospective, multicenter non-interventional, cohort study conducted in HIV-1-infected adult outpatients referred to Italian Departments of Infectious Diseases. This study aimed to evaluate patient satisfaction after switching from PI/r-based treatments to DRV/c in a real-life context by means of two validated questionnaires, the HIV-TSQ and HIV Symptoms Distress Module (HIV-SDM); this latter questionnaire was used to highlight specific clinical symptoms (e.g., gastrointestinal), which were expected to improve as a result of the replacement of ritonavir with cobicistat in ART regimens.
2 Methods
2.1 Study Design
ST.O.RE was a prospective, multicenter, non-interventional cohort study conducted between 2016 and 2018 in Italian HIV-1-infected, adult male and female outpatients, who were currently on a stable ritonavir-boosted antiretroviral (ARV) treatment with PIs (PI/r), either DRV 800 mg once daily or other PIs, for at least 12 months, with demonstrated viral suppression (HIV-RNA < 50 copies/mL) for at least 6 months. Patients were offered to enter this study once their treating physician had considered they were eligible to be administered DRV/c-based treatments [25]. Enrollment was consecutive, and 30% of patients had to be female by the study protocol, to allow for stratified evaluation by gender. Four visits were scheduled during the study: Visit 1 (V1), enrollment; Visit 2 (V2), 4–8 weeks after V1; Visit 3 (V3), 24 ± 6 weeks after V1; Visit 4 (V4), 48 ± 6 weeks after V1.
Patients’ satisfaction for the treatment regimen and current symptoms were evaluated using two validated PRO questionnaires, administered at three timepoints: enrollment (V1), at V2, and at V4. Paper-based versions of the questionnaires were administered, and data were transcribed into an electronic case report form. The study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the ethics committee of each participating center, and all patients provided written informed consent at the time of enrollment. The list of the centers participating in this study is reported in the “Acknowledgments” section.
2.2 Patient-Reported Outcome Questionnaires
The HIV-TSQ and HIV-SDM questionnaires were used during the study. The HIV-TSQ includes ten items with good internal consistency and reliability [26,27,28]. Two versions of this questionnaire were used: at V1, the HIV-TSQ status (HIV-TSQs), whose total score ranges from 0 to 60, with every question ranging from 0 to 6 with higher scores indicating greater treatment satisfaction. At V2 and V4, the administered questionnaire was the HIV-TSQ change (HIV-TSQc), having a total score ranging from − 30 to + 30. Like the HIV-TSQs, a higher score indicated better patient satisfaction: scores < 0 and > 0 indicated a decrease and an increase in treatment satisfaction, respectively. Individual item scores could be considered and reported separately (i.e., very dissatisfied, inconvenient, inflexible, satisfied). As the questionnaires administered at V2 and V4 were the “change” version, they had to be compared with the questionnaires administered at V1, the “status” version [26,27,28]. The questionnaires were defined “evaluable” if they were filled in at the visit and had at least eight out of ten questions answered for each visit [26].
The HIV-SDM questionnaire was developed to assess 20 commonly experienced symptoms, using a rating scale from 0 (“no symptom”) to 4 (“I have the symptom and it bothers me a lot”), thus higher scores would indicate greater symptom distress [29, 30]. Patients were asked about their experiences for each symptom during the past 4 weeks on a five-point Likert scale. The HIV-SDM questionnaires are defined “evaluable” if they have more than 50% of questions answered, otherwise they were excluded from the analysis [29, 30].
2.3 Statistical Analysis
Patient characteristics were described using standard descriptive statistics. Continuous variables were presented as mean values ± standard deviations or median values with 25th–75th percentiles and categorical variables were shown as numbers and percentages.
The questionnaires were analyzed as follows:
HIV-TSQs: in cases where one or two items were missing, the following algorithm was used to calculate the treatment satisfaction score: (1) the existing items score were summed; (2) the sum was divided by the number of existing item scores; and (3) the results were multiplied by ten;
HIV-TSQc: this questionnaire was analyzed as per the HIV-TSQs, except with a scale ranging from − 30 to + 30. To identify statistically significant differences, p values were calculated using the sign test, for the hypothesis that the median of the HIV-TSQc score was equal to 0;
HIV-SDM: all the items were summed to produce a total score ranging from 0 to 80. Higher scores indicated greater symptom distress. Imputations were not performed on missing HIV-SDM data. If patients did not respond, this was interpreted as “never experiencing” the symptom and coded as ‘0’. In the case of more than 50% of missing items, the questionnaires were excluded. As previously described [10], scores were dichotomized to indicate a response (presence of a symptom: scored 1, 2, 3, or 4) or no response (absence of a symptom: scored 0).
Data of PRO total scores for both questionnaires are presented as median with 25th–75th percentiles. Normality of distribution was tested using the Shapiro–Wilk test, resulting in significant (p < 0.001) scores for HIV-TSQs, HIV-TSQc, and HIV-SDM; therefore, non-parametric tests were used. The Wilcoxon rank-sum test was used for comparison between groups (male vs female patients and between previous ARV regimens, namely DRV/r vs other boosted PIs); the sign test was used to analyze HIV-TSQc scores at V2 and V4, HIV-SDM total scores changes were analyzed using a signed-rank test, and changes in the percentage of patients who experienced symptoms between visits were analyzed using the McNemar test or sign test, with the latter used if the number of discordant pairs was ≤ 20. A sensitivity analysis was conducted to evaluate the role of lost to follow-up (i.e., patients who did not follow-up to V4), using the “last observation carried forward” (LOCF) method. Questionnaire scores were analyzed overall and stratified by gender when applicable. A p value of less than 0.05 was considered statistically significant.
3 Results
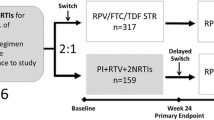
A total of 348 patients were enrolled in the ST.O.RE study of whom 336 were evaluable. Of these, 296 provided both HIV-TSQ and HIV-SDM assessable questionnaires at enrollment (V1) and after 4–8 weeks (V2), while 250 provided both assessable HIV-TSQ and HIV-SDM questionnaires at V1 and at 48 ± 6 weeks (V4). Details are provided in Flow Diagram 1. The main baseline patient characteristics are reported in Table 1.

Among the 336 evaluable patients, 68.2% (229) were male, 94.6% (318) were Caucasian, and 27.3% (92) had hepatitis C virus co-infection. In this cohort, DRV/c was used in a triple-therapy combination in 48.2% of patients, with tenofovir disoproxil fumarate/emtricitabine in 31.5%, abacavir/lamivudine in 16.7%, and dual therapy in 35.2% (mainly with lamivudine). Before the start of DRV/c (and ongoing at enrollment), 81.5% (274/336) of patients were treated with regimens based on DRV/r, 9.8% on ATV/r, 6.3% on LPV/r, and 2.4% on another PI/r. In total, 54 patients withdrew from the study: 23 were lost to follow-up, five withdrew informed consent, four for simplification of regimen, two had confirmed virological failure, two for drug–drug interactions, two for patient choice, one for non-adherence, and 15 for safety reasons. In these latter patients, 21 adverse events (AEs), including one pregnancy, led to study discontinuation; six AEs were not related to DRV/c, three doubtfully, five possibly (of them, two gastrointestinal disorders), one probably (pruritus), and five very likely related (two dyspepsia, two nausea, and one vomiting) [31].
For the HIV-TSQs score, patient satisfaction regarding current ART was already high at enrollment, having a median total score (25th–75th percentiles) equal to 56 (50–60) out of a theoretical maximum of 60 (N = 296). Additionally, the median score of HIV-TSQc was positive during the entire study period, with an improvement observed at V2 (N = 296) and V4 (N = 250), both overall and when stratified by gender. In the overall population, this improved satisfaction was statistically significant at V2 [median (25th–75th percentiles): 26.3 (17–30); N = 296; p < 0.001, sign test] and at V4 [28 (20–30); N = 250; p < 0.001, sign test]. Improved satisfaction was observed within male patients [at V2: 26 (17.5–30), N = 208, p < 0.001; at V4: 27 (19–30), N = 174, p < 0.001; sign test at both visits] and within female patients [at V2: 27 (14.5–30), N = 88, p < 0.001; at V4: 29 (23–30), N = 76, p < 0.001; sign test at both visits]; these results are shown in Fig. 1. No statistically significant differences between genders were observed at V1 (p = 0.17), at V2 (0.65), or at V4 (0.21) [Wilcoxon rank-sum test]. In a sensitivity analysis conducted using the LOCF method for those patients who did not follow up to V4, the median HIV-TSQc scores at V4 were unchanged, i.e., equal to 28 in the overall population, 27 within male patients and 29 within female patients. We also stratified by duration of HIV infection or duration of previous ARV regimen to evaluate if these could influence the questionnaires score but no effect was observed.
Regarding the possible effect of concomitant conditions or medication on questionnaires scores, it was not possible to perform a stratified analysis based on these variables because the prevalence of different comorbidities in this study was low (the most prevalent were: osteopenia, 15.5% overall and hypertension, 15.2% overall). Analyzing the single items, when HIV-TSQs was administered at V1, 195/296 patients (65.9%) reported being “Very satisfied” with their PI/r-based therapy, reporting a “+ 6” score when answering the question “How satisfied are you with your current treatment?” (Fig. 2a). When asked the same question at V2, after 4–8 weeks (N = 296 questionnaires collected), very few patients reported to be slightly less satisfied with their ART (scoring − 1), while most of them reported to be more/much more satisfied with their ART (scoring positive, from + 1 to + 3). At Visit 4 (after 48 weeks, N = 250 questionnaires), further improvements have been observed. Details are provided in Fig. 2a, b (item “Current Treatment”).
When evaluating other items (Fig. 2a, b), the majority of patients expressed a high level of satisfaction at baseline. Nevertheless, further improvement was observed at the following visits (see Fig. 2a, b).
When considering the HIV-SDM scores, the global burden of symptoms decreased throughout the study period. Overall median values were 7.5 (2–17) at V1 (N = 296), 5 (2–14) at V2 (N = 296; p < 0.001, signed-rank test), and 5 (2–13) [N = 250; p = 0.22, signed-rank test] at V4. Median HIV-SDM scores were significantly different between men and women: at V1, 6 (2–13) in male patients vs 13 (3.5–19.5) in female patients (p = 0.012, Wilcoxon rank-sum test), at V2, 5 (1–11) in male patients vs 8 (3–18.5) in female patients (p = 0.009, Wilcoxon rank-sum test), and at V4, 5 (2–10) in male patients vs 8.5 (3–22.5) in female patients (p = 0.002, Wilcoxon rank-sum test).
Considering the specific symptoms, the nine most relevant based on the safety profile of DRV/c were diarrhea, nausea, bloating/pain/gas in stomach, anxiety, skin problems, headache, fatigue, change in body image, and weight loss. Overall, compared with V1, a percentage decrease of patients experiencing symptoms was observed both at V2 and V4, while not being statistically significant for all of them. Notably, a significant decrease both in diarrhea (p = 0.036 at V2 and p = 0.003 at V4, McNemar test) and bloating/gas (p = 0.011 at V2 and p = 0.006 at V4, McNemar test) was observed at both visits. Conversely, a significant increase in nausea was observed both at V2 (p = 0.002, McNemar test) and V4 (p = 0.024, McNemar test). Detailed results are shown in Fig. 3. The same analysis was also stratified by gender, where a similar trend was observed (Fig. 4).
HIV Symptoms Distress Module (HIV-SDM) results stratified by gender for the presence of the most relevant symptoms. Data denoted with “*” were statistically analyzed using the McNemar test. Data denoted by “**” were statistically analyzed using the sign test, performed when the discordant pairs were ≤ 20
To determine if ARV regimens adopted before inclusion in the study could affect symptom burden, we further stratified the HIV-SDM questionnaire scores according to the PI taken before the study entry (DRV/r vs other boosted PIs) (Fig. 5). Notably, when considering the patients coming from DRV/r-based regimens, a significant improvement was observed at V2 (p = 0.001, signed-rank test) and this trend was also observed at V4 (p = 0.054, signed-rank test). The same was not found for patients coming from other PIs (p = 0.29 at V2 and p = 0.34 at V4, signed-rank test) (Fig. 5). It was also found that changes in HIV-SDM total scores between visits were not influenced by the previous PI (p = 0.26 at V2 and p = 0.15 at V4, Wilcoxon rank-sum test).
4 Discussion
ST.O.RE. was the first observational study evaluating the effectiveness and safety of switching from ritonavir-boosted PIs to a DRV/c-based ART and using PROs as a measure of patient satisfaction.
In this prospective, observational, multicenter, non-interventional study, switching from a PI/r to a fixed-dose combination DRV/c-based therapy, with the PI and its booster in a single pill led to an overall increase in patient satisfaction, along with a reduction in symptom burden (particularly gastrointestinal, except for nausea) related to previous regimens as shown by HIV-TSQ and HIV-SDM scores, respectively.
In this study, 296 questionnaires (both HIV-TSQ and HIV-SDM) were collected within V1 and V2 and 250 questionnaires within V1 and V4, which to our knowledge is the highest number of PRO measures obtained in an observational study within the HIV field. Data obtained from the HIV-TSQs showed that baseline patient satisfaction for the current treatment was already fairly high at enrollment, with 66% of patients declaring to be very satisfied with their PI/r-based therapy. After switching to DRV/c, the HIV-TSQc showed further improvement in treatment satisfaction in 88.4% of patients at V4. These findings were further corroborated when examining individual questionnaire items, which ultimately demonstrated a further increase in patient satisfaction.
For HIV-SDM total scores, the overall burden of symptoms decreased from a median value of 7.5 at V1 to 5 at V2 and V4. Stratifying by gender, significant differences were observed between male and female patients, with the latter showing higher scores, indicating a higher burden of symptoms both at V1 and at following visits. These findings further confirm previous observations of a gender effect in female patients, which may be more susceptible to some ART side effects when compared to male patients [32, 33].
This was also confirmed when specific symptoms were examined; it was possible to highlight these differences because of the high percentage of female patients enrolled in this study (30.4%), a figure higher than that usually observed in interventional or observational studies [34]. We found a reduction in the overall population at V4 in percentages of patients experiencing fatigue and gastrointestinal symptoms such as diarrhea and bloating, but not nausea. Notably, gastrointestinal symptoms, usually related to PI/r treatment, may significantly affect patient compliance [35]; these symptoms decreased from V1 to V4, thus showing a clear advantage in terms of tolerability when switching from PI/r-based treatments to DRV/c.
Interestingly, in the present study, only six nausea events were reported as AEs by the clinicians. However, according to HIV-SDM questionnaire results, 40 (13.5% on 296 questionnaires) patients at V2 and 31 (12.4% on 250 questionnaires) patients at V4 reported having experienced nausea. This discrepancy may be explained by the fact that PROs could help patients to better discriminate their symptoms, thus allowing clinicians to obtain data not normally identified through usual clinical practice. Furthermore, when stratifying by gender, female patients showed an increase in nausea and anxiety at V4 while reports of headaches remained stable, whereas all other symptoms decreased, providing further evidence for potential gender effects.
Both HIV-TSQ and HIV-SDM instruments have been used in several recent interventional switching studies [10, 36,37,38]. Of note, most studies reported a lower baseline score for patient satisfaction, presumably because patients were on multi-tablet regimens, and improvements were thus more impressive. Interestingly, in our study, the reduction of just one pill to the new DRV/c-based treatment resulted in increased patient satisfaction as well. Furthermore, adoption of the change (HIV-TSQc) coupled with the status (HIV-TSQs) questionnaires is thought to overcome potential ceiling effects of HIV-TSQs, as shown in both our study and in previous switching studies [38].
Even though these instruments are recognized tools used to evaluate patient satisfaction, they are mainly used in the research studies in different ways. For instance, the relatively new change version of HIV-TSQ is less widely adopted than the baseline status version. This might be owing to the possibly confusing scores resulting from different scales (0–60 vs − 30/+ 30), which could affect interpretation and results comprehension. In addition, a definitive consensus on the use of the available validated questionnaires has not been reached yet. Put into perspective, it should be highlighted how the therapeutic alliance between patients and clinicians plays a key role in the context of chronic disease and lifelong therapy, such as that of HIV-infected patients, and this study aimed to transpose PRO experiences acquired from HIV interventional trials into a real-life study. The new motto of the ART era can be expressed as “beyond viral suppression” because QoL has been shown to be relatively high in patients with HIV but still not as high as those without HIV [39]. Taking up the challenge posed by Lazarus et al. [4] in ensuring that 90% of virally suppressed patients have good health-related quality of life can be disruptive with respect to how healthcare practitioners interact with patients with HIV. Patient-reported outcomes have been shown to have a higher correlation with patient health status when compared to what clinicians reported [40, 41]. This was highlighted in the present study, which showed that PROs helped identify symptoms and changes in patient satisfaction after starting a new treatment regimen, better than that usually achieved without questionnaire use. Notably, HIV-specific PROs reporting perceived AEs in clinical practice, together with laboratory data and AEs reported by clinicians, represent useful tools to holistically evaluate HIV-infected patients. Patient-reported outcomes allow clinicians to more deeply analyze patient satisfaction and tolerability of the current therapy and, as a result, high reported scores could lead to increased treatment adherence. The limitations of this study include the non-randomized, open-label, single-arm design. Moreover, enrolled patients were mainly Caucasian, thus the results may not necessarily be representative of other target populations. Patient-reported outcome use may have helped clinicians obtain more data on ART tolerability; however, we could hypothesize that patients underwent a “drucebo” effect and were influenced to report symptoms as a result of being asked about a particular symptom [42]. Furthermore, regarding a possible “Hawthorne effect” (i.e., the possible impact on behavior in patients aware of being studied), it should be pointed out that bias could not be 100% avoided because the patients had to be informed and had to sign an informed consent before study entrance and questionnaire filling. Furthermore, this effect is common and not different from what occurs in other clinical studies [43]. The questionnaires were filled by the patients outside the visiting room, without the presence of clinicians; the questionnaires were anonymous, thus patients’ privacy was respected. This study also showed that a DRV/c based-ART is still an optimal therapeutic option in a wide range of patients, because in addition to its well-known high genetic barrier to resistance, useful for patients with sub-optimal adherence [44], it entails a clear improvement in the satisfaction of patients with HIV.
5 Conclusions
Our study demonstrated further improvements in patient-reported satisfaction, even when taking into consideration the already high baseline patient satisfaction scores, suggesting that DRV/c could represent a good switching option for patients with stable HIV currently in therapy with PI/r. Patient-reported outcomes were shown to be valuable for evaluating patient satisfaction and treatment tolerability; ultimately, in view of achieving the “fourth 90”, wider use of these instruments in observational studies and clinical practice could help achieve global benchmarks regarding HIV control programs.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
29 May 2020
In the original version of this article, in Fig.��2b the formatting on the x-axis of the graph has been published incorrectly.
References
Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–56.
O’Connor J, Smith C, Lampe FC, et al. Durability of viral suppression with first-line antiretroviral therapy in patients with HIV in the UK: an observational cohort study. Lancet HIV. 2017;4:e295–302.
Sheth AN, Ofotokun I, Buchacz K, et al. Antiretroviral regimen durability and success in treatment-naive and treatment-experienced patients by year of treatment initiation, United States, 1996–2011. J Acquir Immune Defic Syndr. 2016;71:47–56.
Lazarus JV, Safreed-Harmon K, Barton SE, et al. Beyond viral suppression of HIV: the new quality of life frontier. BMC Med. 2016;14:94.
Webster P. UNAIDS survey aligns with so-called fourth 90 for HIV/AIDS. Lancet. 2019;393:2188.
Clay PG, Nag S, Graham CM, Narayanan S. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine (Baltimore). 2015;94:e1677.
Domingo P, Mateo MG, Gutierrez MDM, Vidal F. Tolerability of current antiretroviral single-tablet regimens. AIDS Rev. 2018;20:141–9.
Toupin I, Engler K, Lessard D, et al. Developing a patient-reported outcome measure for HIV care on perceived barriers to antiretroviral adherence: assessing the needs of HIV clinicians through qualitative analysis. Qual Life Res. 2018;27:379–88.
Kjær ASHK, Rasmussen TA, Hjollund NH, Rodkjaer LO, Storgaard M. Patient-reported outcomes in daily clinical practise in HIV outpatient care. Int J Infect Dis. 2018;69:108–14.
Brunetta J, Moreno Guillén S, Antinori A, et al. Patient-reported outcomes after a switch to a single-tablet regimen of rilpivirine, emtricitabine, and tenofovir DF in HIV-1-positive, virologically suppressed individuals: additional findings from a randomized, open-label, 48-week trial. Patient. 2015;8:257–67.
Tashima K, Crofoot G, Tomaka FL, et al. Cobicistat-boosted darunavir in HIV-1-infected adults: week 48 results of a phase IIIb, open-label single-arm trial. AIDS Res Ther. 2014;11:39.
Marzolini C, Gibbons S, Khoo S, Back D. Cobicistat versus ritonavir boosting and differences in the drug-drug interaction profiles with co-medications. J Antimicrob Chemother. 2016;71:1755–8.
Eron JJ, Orkin C, Gallant J, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32:1431–42.
Orkin C, Molina JM, Negredo E, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5:e23–34.
Lathouwers E, Wong EY, Luo D, Seyedkazemi S, De Meyer S, Brown K. HIV-1 resistance rarely observed in patients using darunavir once-daily regimens across clinical studies. HIV Clin Trials. 2017;18:196–204.
Mallolas J. Darunavir stands up as preferred HIV protease inhibitor. AIDS Rev. 2017;19:105–12.
Capetti A, Cossu MV, Rizzardini G. Darunavir/cobicistat for the treatment of HIV-1: a new era for compact drugs with high genetic barrier to resistance. Expert Opin Pharmacother. 2015;16:2689–702.
Deeks ED. Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs. 2014;74:195–206.
Murray M, Hopking J, Sievers J, et al. Improvements in patient reported outcomes of dolutegravir (DTG) based second-line treatment compared to lopinavir/ritonavir (LPV/r) based treatment: results from the DAWNING study [abstract]. JIAS. 2018;21(Suppl. 8):105.
Oglesby A, Angelis A, Punekar Y, et al. Patient-reported outcomes after switching to a 2-drug regimen of dolutegravir + rilpivirine: week 100 results from the SWORD-1 and SWORD-2 Studies. JIAS. 2018;21(Suppl. 8):101.
Benson C, Simonson R, Bicer C, Dunn K. High levels of patient satisfaction during rapidly initiated therapy with darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) for treatment of HIV-1 infection through 24 weeks of the DIAMOND study [abstract]. JIAS. 2018;21(Suppl. 8):49.
Wohl D, Clarke A, Maggiolo F, et al. Patient-reported symptoms over 48 weeks among participants in randomized, double-blind, phase III non-inferiority trials of adults with HIV on co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus co-formulated abacavir, dolutegravir, and lamivudine. Patient. 2018;11(5):561–73.
Black N. Patient reported outcome measures could help transform health care. BMJ. 2013;346:f167.
Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: a new era in clinical research. Perspect Clin Res. 2011;2:137–44.
European Medicines Agency. Summary of Rezolsta product characteristics. https://www.ema.europa.eu/en/documents/product-information/rezolsta-epar-product-information_en.pdf. Accessed 16 Mar 2020.
HIV-TSQ guidelines. https://www.healthpsychologyresearch.com/sites/default/files/guidelines/HIVTSQ%20Summary_rev.11.8.15.pdf. Accessed 16 Mar 2020.
Woodcock A, Bradley C. Validation of the HIV treatment satisfaction questionnaire (HIVTSQ). Qual Life Res. 2001;10(6):517–31.
Woodcock A, Bradley C. Validation of the revised 10-item HIV Treatment Satisfaction Questionnaire status version and new change version. Value Health. 2006;9(5):320–33.
Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54(Suppl. 1):S77–90.
Marc LG, Wang MM, Testa MA. Psychometric evaluation of the HIV symptom distress scale. AIDS Care. 2012;24(11):1432–41.
Gori A, Antinori A, Ripamonti D, et al. Effectiveness and safety of cobicistat-boosted darunavir-based antiretroviral treatment in an Italian observational cohort: the TMC114FD1HTX4003 (ST.O.RE) study [abstract]. JIAS. 2018;21(Suppl. 8):109.
Kempf MC, Pisu M, Dumcheva A, Westfall AO, Kilby JM, Saag MS. Gender differences in discontinuation of antiretroviral treatment regimens. J Acquir Immune Defic Syndr. 2009;52:336–41.
Scully EP. Sex differences in HIV infection. Curr HIV AIDS Rep. 2018;15:136–46.
Curno MJ, Rossi S, Hodges-Mameletzis I, Johnston R, Price MA, Heidari S. A systematic review of the inclusion (or exclusion) of women in HIV research: from clinical studies of antiretrovirals and vaccines to cure strategies. J Acquir Immune Defic Syndr. 2016;71:181–8.
Swan H, Reisman JI, McDannold SE, et al. The relationship between gastrointestinal symptom attribution, bothersomeness, and antiretroviral adherence among adults with HIV. AIDS Care. 2018;30(8):997–1003.
Huhn GD, Tebas P, Gallant J, et al. A randomized, open-label trial to evaluate switching to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide plus darunavir in treatment-experienced HIV-1-infected adults. J Acquir Immune Defic Syndr. 2017;74:193–200.
Wilkins EL, Cohen CJ, Trottier B, et al. Patient-reported outcomes in the single-tablet regimen (STaR) trial of rilpivirine/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate in antiretroviral treatment-naive adults infected with HIV-1 through 48 weeks of treatment. AIDS Care. 2016;28:401–8.
Overton ET, Orkin C, Swindells S, et al. Monthly long-acting cabotegravir and rilpivirine is non-inferior to oral ART as maintenance therapy for HIV-1 infection: week 48 pooled analysis from the phase 3 ATLAS and FLAIR studies. In: The 10th IAS Conference on HIV Science; July 21–24, 2019; Mexico City, MX (Abstract 1291).
Zeluf-Andersson G, Eriksson LE, Schönnesson LN, Höijer J, Månehall P, Ekström AM. Beyond viral suppression: the quality of life of people living with HIV in Sweden. AIDS Care. 2019;31:403–12.
Fares CM, Williamson TJ, Theisen MK, et al. Low concordance of patient-reported outcomes with clinical and clinical trial documentation. JCO Clin Cancer Inform. 2018;2:1–12.
Marando F, Gualberti G, Costanzo AM, et al. Discrepancies between physician’s perception of depression in HIV patients and self-reported CES-D-20 assessment: the DHIVA study. AIDS Care. 2016;28:147–59.
Penson PE, Mancini GBJ, Toth PP, et al. Introducing the ‘Drucebo’ effect in statin therapy: a systematic review of studies comparing reported rates of statin-associated muscle symptoms, under blinded and open-label conditions. J Cachexia Sarcopenia Muscle. 2018;9:1023–33.
McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–77.
Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14:49–59.
Acknowledgements
We thank all the patients and all the clinicians participating in this study: INMI “L. Spallanzani”, Roma; A.O.U. Careggi, Firenze; A.O.U. Policlinico Giaccone, Palermo; ASST Spedali Civili, Brescia; Fondazione Policlinico A. Gemelli, Roma; A.O. Pugliese Ciaccio, Catanzaro; A.O.U. Senese, Siena; Ospedale Amedeo di Savoia, Torino; ASST Fatebenefratelli Sacco, 1st and 3rd Divisions of Infectious Diseases, Milano; P.O. Cotugno, Napoli; ASST San Gerardo, Monza; Ospedale San Raffaele, Milano; Fondazione IRCCS Policlinico S. Matteo, Pavia, A.O.U. Sassari, Sassari; Ospedale Amedeo di Savoia, Torino; EO Ospedali Galliera, Genova; Ospedale di Circolo, Busto Arsizio; ASST Papa Giovanni XXIII, Bergamo; Policlinico Umberto I, Roma; A.O.U. Ospedali Riuniti, Foggia; A.O.U. Padova, Padova; ARNAS Garibaldi, Catania; ASST Santi Paolo e Carlo, Milano; Policlinico Tor Vergata, Roma.
Author information
Authors and Affiliations
Contributions
DM, AU, RT, AG, and AA designed the study and contributed to study protocol finalization. DM, RT, and AU wrote the study protocol and reviewed the statistical analyses; FR reviewed the statistical analyses. DM and FR wrote the manuscript. RT reviewed, commented, and approved the manuscript; AU reviewed and approved the manuscript. AA, MVC, BM, GS, NS, VDC, AC, EF, AC, GO, DV, GDE, LA, SF, MEL, GM, EP, PS, BR, and AG enrolled the patients, reviewed the statistical analyses, reviewed the manuscript, provided their comments, and approved this manuscript.
Corresponding author
Ethics declarations
Funding
This study was funded by Janssen-Cilag SpA.
Conflict of interest
Andrea Antinori received a grant from Gilead Sciences, ViiV Healthcare, and Janssen-Cilag; he received consulting fees or honorarium from ViiV Healthcare, Janssen-Cilag, and Merck. Emanuele Foca has received consultancy fees and honoraria by several pharmaceutical companies in the field of HIV infections. Annamaria Cattelan received a grant from Janssen-Cilag and consulting fees or honorarium from ViiV Healthcare, MSD, Gilead Sciences, and Janssen-Cilag. Giordano Madeddu received consulting fees or honorarium from ViiV Healthcare, Gilead Sciences, MSD, and Janssen-Cilag; he received a speaker’s fee from ViiV Healthcare, Angelini, and Pfizer. Emanuele Pontali received consulting fees or honorarium for advisory board participation for Janssen-Cilag; he received payments for training activities for Janssen-Cilag. Barbara Rossetti received consulting fees or honorarium for educational activities with REPs and a support for meeting participation. Andrea Gori received a grant from Janssen-Cilag, ViiV Healthcare, MSD, BMS, Abbvie, Gilead Sciences, Novartis, Pfizer, Astellas, AstraZeneca, and Angelini; he received consulting fees or honorarium from Janssen-Cilag, ViiV Healthcare, MSD, BMS, Abbvie, Gilead Sciences, Novartis, Pfizer, Astellas, AstraZeneca, and Angelini. Maria V. Cossu, Barbara Menzaghi, Gaetana Sterrantino, Nicola Squillace, Valentina Di Cristo, Antonella Catagna, Giancarlo Orofino, Daniela Valenti, Gabriella D’Ettore, Lucia Aprea, Sergio Ferrara, Maria E. Locatelli, Paolo Scerbo, and Francesco Rucci have no conflicts of interest that are directly relevant to the content of this article. Alessia Uglietti, Roberta Termini, and Daniela Mancusi are Janssen employees.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Antinori, A., Cossu, M.V., Menzaghi, B. et al. Patient-Reported Outcomes in an Observational Cohort of HIV-1-Infected Adults on Darunavir/Cobicistat-Based Regimens: Beyond Viral Suppression. Patient 13, 375–387 (2020). https://doi.org/10.1007/s40271-020-00413-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-020-00413-y