Abstract
Background
European guidelines advocate the measurement of on-treatment hepatitis C virus (HCV) RNA in order to determine optimal therapy duration (response-guided therapy [RGT]) in patients with rapid virological response (RVR) or delayed virological response (DVR). Treatment response is highly dependent upon the extent of liver fibrosis yet there is little evidence quantifying the cost effectiveness of RGT particularly conditional upon fibrosis stage.
Objective
This study describes an economic model designed to assess the costs and benefits of RGT compared with standard duration of therapy (SDT) in hepatitis C virus genotype 1 patients.
Methods
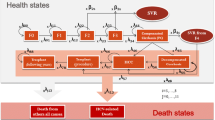
A Markov cohort simulation model with lifetime perspective was developed to undertake a cost utility analysis of RGT in the UK. Patients entered the model at Metavir disease stages F0–F4, and progressed through these stages via age and duration of HCV infection-dependent transition probabilities. Treated patients were partitioned according to virological response and shortened or extended duration of therapy was applied following European guidelines.
Results
For all patients, SDT and RGT was associated with an increase of 2.14 and 2.20 QALYs and £2,374 and £2,270 costs, respectively, compared with no treatment. Overall, RGT was a dominant scenario being associated with a lower risk of complications, increased QALYs (0.08) and cost saving (£101). RGT across fibrosis stages was either highly cost effective or dominant; in all cases RGT was associated with an increase in QALYs, driven by a reduction in complications in DVR subjects and reduced exposure to treatment disutility in RVR subjects; costs were lower in F1 and F2 fibrosis stages. At a willingness-to-pay threshold of £20,000 per QALY, overall RGT across fibrosis stages F2–F4 were associated with the highest probability of being cost effective. At this threshold, the probability of reduced/extended therapy in RVR/DVR patients being cost effective is 0.35 and 0.88, respectively.
Conclusions
This analysis suggests that the treatment of HCV genotype 1 patients in fibrosis stage F2 has the greatest potential for maximizing health benefit and cost saving within an RGT protocol. Predicting those patients most likely to respond to treatments is important from both a clinical and cost perspective and the tailoring of treatment duration with the current standard of care is likely to remain a priority for payers with budgetary constraints.




Similar content being viewed by others
References
Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81.
Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–67.
Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46.
Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer. 2008;98(7):1166–75.
Calvaruso V, Craxì A. 2011 European Association of the Study of the Liver hepatitis C virus clinical practice guidelines. Liver Int. 2012;32(Suppl 1):2–8.
Harris RJ, Ramsay M, Hope VD, Brant L, Hickman M, Foster GR, et al. Hepatitis C prevalence in England remains low and varies by ethnicity: an updated evidence synthesis. Eur J Public Health. 2012;22(2):187–92.
Health Protection Agency. Hepatitis C in the UK: 2011 Report. London: Health Protection Agency; 2011.
Schlütter J. Therapeutics: new drugs hit the target. Nature. 2011;474(7350):S5–7.
Sroczynski G, Esteban E, Conrads-Frank A, Schwarzer R, Mühlberger N, Wright D, et al. Long-term effectiveness and cost-effectiveness of antiviral treatment in hepatitis C. J Viral Hepat. 2010;17(1):34–50.
Nakamura J, Kobayashi K, Toyabe SI, Aoyagi Y, Akazawa K. The cost-effectiveness of the new protocol reflecting rapid virologic response to peginterferon alpha-2b and ribavirin for chronic hepatitis C. Eur J Gastroenterol Hepatol. 2007;19(9):733–9.
Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15(17):i–xii, 1–210.
Nakamura J, Toyabe SI, Aoyagi Y, Akazawa K. Economic impact of extended treatment with peginterferon alpha-2a and ribavirin for slow hepatitis C virologic responders. J Viral Hepat. 2008;15(4):293–9.
Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48(2):418–31.
Sullivan SD, Craxi A, Alberti A, Giuliani G, De Carli C, Wintfeld N, et al. Cost effectiveness of peginterferon alpha-2a plus ribavirin versus interferon alpha-2b plus ribavirin as initial therapy for treatment-naive chronic hepatitis C. Pharmacoeconomics. 2004;22(4):257–65.
Sullivan SD, Jensen DM, Bernstein DE, Hassanein TI, Foster GR, Lee SS, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol. 2004;99(8):1490–6.
Mangia A, Andriulli A. Tailoring the length of antiviral treatment for hepatitis C. Gut. 2010;59(1):1–5.
Pearlman BL. Extended-therapy duration for chronic hepatitis C, genotype 1: the long and the short of it. World J Gastroenterol. 2008;14(23):3621–7.
Cheng WSC, Roberts SK, McCaughan G, Sievert W, Weltman M, Crawford D, et al. Low virological response and high relapse rates in hepatitis C genotype 1 patients with advanced fibrosis despite adequate therapeutic dosing. J Hepatol. 2010;53(4):616–23.
Joint Formulary Committee. British National Formulary. 60th ed. London: British Medical Association and Royal Pharmaceutical Society of Great Britain; 2010.
Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon alpha-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8(39):iii–iv, 1–125.
Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11(11):1–205, iii.
Martin NK, Vickerman P, Miners A, Foster GR, Hutchinson SJ, Goldberg DJ, et al. Cost-effectiveness of hepatitis C virus antiviral treatment for injection drug user populations. Hepatology. 2012;55(1):49–57.
Clark PJ, Thompson AJ, McHutchison JG. IL28B genomic-based treatment paradigms for patients with chronic hepatitis C infection: the future of personalized HCV therapies. Am J Gastroenterol. 2011;106(1):38–45.
Alberti A. Impact of a sustained virological response on the long-term outcome of hepatitis C. Liver Int. 2011;31(Suppl 1):18–22.
Ciesek S, Manns MP. Hepatitis in 2010: the dawn of a new era in HCV therapy. Nat Rev Gastroenterol Hepatol. 2011;8(2):69–71.
Acknowledgments
We would like to thank Sunita Nair and Mahendra Rai, Cardiff Research Consortium, for providing editorial support to the manuscript.
Author contributions
PM constructed the model, contributed to the design of the study, undertook the analysis and wrote the first draft. YY contributed to the study and model design and analysis, contributed to subsequent drafts. RK designed the study, contributed to the model design and subsequent drafts. All authors approved the final version. PM is the guarantor for the overall content.
Conflict of interest
This study was funded by, and the writing of the manuscript supported by, an unrestricted grant from Bristol-Myers Squibb. YY is an employee of Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McEwan, P., Kim, R. & Yuan, Y. Assessing the Cost Utility of Response-Guided Therapy in Patients with Chronic Hepatitis C Genotype 1 in the UK Using the MONARCH Model. Appl Health Econ Health Policy 11, 53–63 (2013). https://doi.org/10.1007/s40258-012-0002-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-012-0002-0