Abstract
Pure and doped catalysts were prepared with ZnO dopant concentration (0.04–0.08 mol) by wet impregnation method followed by calcination at 350–650 °C. The prepared solids were characterized by X-ray diffraction, N2-adsorption at −196 °C and the methanol conversion as the catalytic probe reaction. The results revealed that the crystallite size and ordering of CuO phase decrease by ZnO-doping for samples calcined at 350–550 °C. Opposite trends were observed by increasing ZnO amount to 0.08 mol (i.e. 9.21 wt%). The specific surface area (S BET) and the catalytic activity of pure catalyst increase by increasing the calcination temperature to 550 °C and/or increasing the amounts of ZnO up to certain extent reaching to a maximum at 7.07 wt% ZnO; above this concentration catalytic activity of doped samples decreases. But at calcination temperature above 550 °C, the catalytic activity and selectivity decrease. The prepared catalysts are selective towards formaldehyde and methyl formate formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methanol has been considered as an important raw material in the synthesis of various chemicals. Dehydrogenation of methanol was expected as a promising process to synthesize formaldehyde and methyl formate [1]. Formaldehyde is an industrial chemical that is widely used to manufacture numerous household products [2]. Formaldehyde and methyl formate are produced in large quantity by the coal chemical industry and natural gas chemical industry [3], both of them are important organic chemical materials usually used as the raw material of medicines [4]. Methanol is also considered as a candidate for the chemical carrier to transport hydrogen [5]. Copper-based catalysts appeared to be active towards the dehydrogenation of methanol. The activity and selectivity of catalyst were strongly influenced by both reaction conditions and surface structure. On reduced copper surface, CH3O species decomposed near 370 K to form formaldehyde and methyl formate [6]. Over the past two decades, there has been considerable interest in the adsorption and decomposition mechanism of methanol over solid surfaces such as Cu, Pt, Ni, TiO2 and other oxides [7–11].
Improving the catalytic activity and selectivity of metal oxides employed in some important industrial reactions is achieved using suitable support, exposure to radiation and doping with certain foreign cations [12, 13]. Doping of single or multicomponent metal or metal oxide system with certain foreign oxides was efficient approach for bringing about significant modifications in their thermal stability, electrical, optical, magnetic, surface and catalytic properties [14]. The addition of small amounts of certain foreign cations such as Li+, Zr4+ and K+ to CuO/MgO has influenced the mutual solid–solid interaction between constituents [15, 16]. This influence may be accompanied by significant changes in the catalytic and physicochemical properties of the doped catalysts [14]. Doping CuO/MgO system with K2O decreased the degree of ordering of CuO and MgO phases [16]. Li2O-doping of CuO/MgO solid enhanced its catalytic activity towards conversion of iso-propanol [15, 17]. ZnO is a well-known dopant for many catalytic systems such as NiO and Co3O4, in which ZnO brought about measurable changes in their specific surface areas and the crystallite sizes of their phases [18]. Doping Ni–Al2O3 system with ZnO affected on the range of reduction temperature of NiO due to formation of ZnAl2O4 spinel-liked structure [19]. It was reported that the reactivity of Fe2O3 to interact with MO (M = Mg, Ni, Co and Mn) yielding the corresponding MFe2O4 had been found to be much stimulated by doping with ZnO [20, 21]. Zn2+ had a high activity to diffuse into Co3O4 and Fe2O3 solids at about 600 °C [22]. Doping CuO/TiO2 with ZnO brought about a measurable decrease in the crystallite size of both anatase and CuO phases to an extent proportional to the amount of dopant added [23].
The present work aimed at studying the role of calcination temperature and ZnO doping of CuO/MgO system was prepared by impregnation method on its structural, surface characteristics, catalytic activity and selectivity. The techniques employed were X-ray-diffraction, N2-adsorption at −196 °C.
Experimental
Materials
A known amount of magnesium hydroxide (previously prepared [24]) was impregnated with a solution containing a known amount of copper nitrate dissolved in the least amount of distilled water. The CuO content in all solid samples was fixed at 23 mol%. The solutions prepared contained different proportions of Zn (NO3)2·6H2O. The obtained pastes were dried at 110 °C and then calcined at 350–650 °C for 4 h. The amount of zinc oxide expressed in mole was 0.04, 0.05, 0.06 and 0.08. The undoped sample was nominated as CuMgO, but doped solids were determined and nominated as 0.04, 0.05, 0.06, 0.08 ZnO-doped CuMgO.
Techniques
X-Ray diffractograms of various prepared solids were determined using a Bruker diffractometer (Bruker D8 Advance). The scanning rate was fixed at 8° in 2θ/min and 0.8° in 2θ/min for phase identification and line broadening analysis, respectively. The patterns were run with CuKα1 with secondly monochromator, λ = 0.15005 nm at 40 kV and 40 mA. The crystallite size of the phases present was calculated using Scherrer equation [25].
The specific surface areas of the solid catalyst samples were determined from nitrogen adsorption desorption isotherms measured at −196 °C using a Quanta chrome NOVA 2000 automated gas-sorption apparatus model 7.11. All samples were degassed at 200 °C for 2 h under a reduced pressure of 10−5 Torr before undertaking such measurements.
The catalytic activities of pure and variously ZnO doped-CuMgO solid catalyst samples towards methyl alcohol dehydrogenation were determined at reaction temperatures 125–275 °C, the catalytic reaction being conducted in a flow reactor under atmospheric pressure. Thus, a 50 mg catalyst sample was held between two glass wool plugs in a Pyrex glass reactor tube 20 cm long and 1 cm internal diameter packed with quartz fragments 2–3 mm length. The temperature of the catalyst bed was regulated and controlled to within ±1 °C. Argon gas was used as the diluents and the methyl alcohol vapor was introduced into the reactor through an evaporator/saturator containing the liquid reactant at constant temperature 26 °C. The flow rate of the carrier gas was maintained at 15 ml/min. Before carrying out such catalytic activity measurements, each catalyst sample was activated by heating at 300 °C in a current of argon for 1 h then cooled to the catalytic reaction temperature. The injection time of the reaction products and the unreacted methyl alcohol was fixed after 15 min, and GC control at t = constant was performed until achieving a steady state process. The reaction products in the gaseous phase were analyzed chromatographically using Perkin-Elmer Auto System XL Gas Chromatograph fitted with a flame ionization detector. Stainless steel chromatographic columns were used, 4 m length, packed with 10 % squalane supported on chromosorp. The reaction products were analyzed at a column temperature of 40 °C in all conversion runs. Detector temperature was kept at 250 °C.
Results and discussion
XRD investigation of pure ZnO-doped CuMgO solids calcined at different temperatures
X-Ray diffractograms of pure and variously ZnO-doped solids precalcined at 350–650 °C were determined and illustrated in Figs. 1 and 2. The values of crystallite sizes of different phases were calculated using Scherrer equation [25], are given in Table 1. Inspection of Figs. 1 and 2 and Table 1 revealed that: (1) all investigated solids precalcined at 350, 550 °C consisted of MgO as a major phase, CuO as a minor thereof together with Cu2O phase in nanocrystalline nature. The presence of Cu2O phase was evidenced from the brown color of the calcined solids beside presence of diffraction line at d = 2.44 Å (at I/I > 10 c/o). (2) Doping CuMgO system with 0.06 mol ZnO (i.e. 7.07 wt%) brought about a measurable decrease in both of ordering and crystallite sizes of MgO and CuO phases, further increase in ZnO amount to 0.08 mol (i.e. 9.21 wt%) resulted in an increase in the degree of ordering of CuO phase while opposite result was found in case of MgO phase. (3) Calcination at 350 and 550 °C of the doped solids resulted in the formation of nanocrystalline ZnO phase, its crystallite size increases with increasing ZnO content. (4) The rise in calcination temperature of pure and ZnO-doped solids to 650 °C causes decreasing the degree of ordering of CuO, MgO and ZnO phases, but led to increasing the crystallite size of CuO (4.7–25 nm).
These obtained results can be explained in the role of ZnO-doping in increasing the degree of dispersion of active phase [26, 27] via decreasing its crystallite sizes. The mechanism of this action had been tentatively attributed to coating the copper oxide crystallites by ZnO film that may hinder the particle adhesion of the doped oxide solids [26]. The re-increase of degree of crystallinity of CuO phase in the highly doped sample (0.08 mol) at 550 °C could be due to the presence of maximum limit for ZnO to hinder the grain growth of CuO. Increasing ZnO content to 0.06 mol at 650 °C led to dissolving ZnO in MgO and forms solid solution; this interaction led the finally divided CuO crystallites to grow on the top surface layer of MgO. This speculation was based on the effective increase in the crystallite size of CuO as shown in Table 1.
Specific surface areas of pure and ZnO-doping CuMgO calcined at different temperatures
The surface characteristics of pure adsorbent sample and those treated with small amounts of ZnO precalcined in air at 550 and 650 °C were determined from nitrogen adsorption isotherms conducted at −196 °C. The dopant concentrations were 0.06 and 0.08 mol ZnO per mol MgO corresponding to 7.07 and 9.21 wt%, respectively. The surface characteristics, namely S BET, V p “total pore volume” and r − “mean pore radius” calculated for various adsorbents are listed in Table 2. Inspection of the results in Table 2 shows that (1) addition of ZnO resulted in a progressive increase in the specific surface areas of the treated solids. The maximum increase in the S BET due to treatment with 0.06 and 0.08 mol ZnO attained about 33 and 52 % for the solids calcined at 550 °C. The observed increase in the specific surface areas of ZnO-doped solids may be attributed to formation of new pores. The formation of these pores may be due to liberation of gaseous nitrogen oxides in the course of the heat treatment of zinc nitrate added. (2) Increasing the calcination temperature from 550 to 650 °C decreases the specific surface areas of the treated solids. The decrease in the S BET by increasing calcination temperature to 650 °C attained 24 and 21 % for pure and doped solid with 0.06 mol ZnO, respectively. This decrease in specific surface areas of the treated solids may be attributed to collapse of the pore structure and/or the particle adhesion process (grain growth). In fact, the BET-surface area of CuMgO treated with 0.06 mol ZnO conducted at 650 °C measured 22 m2/g while the ZnO-free sample calcined at the same temperature measured 16 m2/g. These findings might suggest that ZnO acted as convenient stabilizer against thermal sintering process of the treated solids.
The extension in the surface area due to ZnO-doping is expected to be accompanied by a corresponding improvement in their catalytic activity.
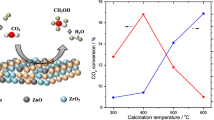
Catalytic activity and selectivity of the pure and ZnO-doped CuMgO systems towards methanol dehydrogenation reactions
Doping with small amounts of ZnO affected both structural and surface properties of the investigated CuMgO system. The changes in structure and/or the surface can reflect the catalytic properties of these solid catalysts. The effect of ZnO–doping (0.04–0.08 mol) corresponding to (4.83–9.21 wt%) of CuMgO followed by calcinations at 350–650 °C on the catalytic activity and selectivity was investigated at different reaction temperatures (125–275 °C) as shown in Figs. 3 and 4 and Tables 3 and 4. The reaction proceeded via dehydrogenation to give both methyl formate and formaldehyde [28]. Examination of Figs. 3 and 4 and Tables 3 and 4 shows the following: (1) the catalytic activity of investigated solids (expressed as conversion of methanol) increases with increasing reaction temperature in the range of 125–175 °C. Further increase in the reaction temperature is followed by a small decrease in the catalytic activity (above this temperature the activity tends to be stable). (2) Doping CuMgO solid with ZnO from 0.04 to 0.06 mol in the range of 125–175 °C led to increasing the conversion and selectivity of both formaldehyde and methyl formate. But increasing reaction temperature above 175 °C led to small increase in the conversion of methanol. (3) Further increase in ZnO content to (0.08 mol) decreases the conversion of methanol. (4) The catalytic activity and selectivity of pure and ZnO-doped solids increased with increasing the calcination temperature from 350 to 550 °C, but above this temperature the catalytic activity and selectivity decrease. (5)The investigated solids are selective to formaldehyde (S F %) at low temperature, this selectivity decreases with increasing reaction temperature from 125 to 275 °C. The selectivity towards methyl formate (S m %) was more pronounced at high reaction temperature.
The results mentioned above can be explained in the light of: (1) the investigated CuMgO system is dehydrogenation catalyst. The prepared catalysts are selective to formaldehyde formation due to the presence of dehydrogenation sites (copper species) [15, 29–31]. (2) The observed increase in the catalytic activity and selectivity of solid samples doped with ZnO (0.06 mol) 7.07 wt% may be attributed to an affective increase in the concentration of active sites involved in the catalytic reaction via decreasing the crystallite size of CuO and MgO phase (as shown in “XRD” section), beside increasing the S BET as shown in Table 2 and the presence of ZnO as dehydrogenation catalyst. (3) Decreasing the catalytic activity and selectivity of doped solids above 0.06 mol% ZnO may be due to decreasing the ability of ZnO to hinder the grain growth of CuO and small amount of ZnO dissolves in MgO matrix. These effects yielded big crystallites of CuO (as shown in “XRD” section). (4) The observed increase in the catalytic activity by increasing the calcination temperature from 350 to 550 °C can be explained in the light of possible completeness of thermal decomposition of Mg(OH)2 and also formation of new active sites responsible for increasing the catalytic activity, beside increasing the S BET as shown in Table 2. (5) Beside the possible dissolution of ZnO in both CuO and MgO lattice yielding various solid solutions [32], the obtained decrease in the catalytic activity as a result of increasing the calcination temperature above 550 °C may be attributed to an effective increase in the crystallite size of copper oxides in pure and doped solids (as shown in “XRD” section), which was evidenced also, by decreasing the S BET as shown in Table 2. The portion of CuO and/or ZnO involved in solid solution should have very small catalytic activity.
Conclusions
In conclusion, the physicochemical and catalytic properties of CuMgO system are affected by ZnO-doping and calcination temperature. The results revealed that:
-
1.
The crystallite size and ordering of CuO phase decrease to (4 nm) by ZnO-doping (<0.08 mol) for samples calcined at 350–550 °C. Opposite trends was observed by increasing ZnO amount to 0.08 mol (i.e. 9.21 wt%).
-
2.
The BET surface area and catalytic activity of CuMgO catalyst increase by increasing the calcination temperature from 350 to 550 °C and/or by increasing the amounts of ZnO up to certain extent reaching to a maximum at 7.07 wt% ZnO.
-
3.
In the pure and doped samples, increasing the calcination temperature to 650 °C led to increasing the crystallite size of CuO, decreasing the catalytic activity and selectivity.
-
4.
The prepared catalysts were selective towards formaldehyde and methyl formate formation.
References
Guerrero-Ruiz A, Rodriguez-Ramos I, Fierro JLG (1991) Dehydrogenation of methanol to methyl formate over supported copper catalysts. Appl Catal 72:119
Thorud S, Gjolstad M, Ellingsen DG, Molander P (2005) Air formaldehyde and solvent concentrations during surface coating with acid-curing lacquers and paints in the woodworking and furniture industry. J Environ Monit 7:586
Lee JS, Kim JC, Kim YG (1990) Methyl formate as a new building block in C1 chemistry. Appl Catal 57:1
Lee YS, Kim JC, Lee JS, Kim YG (1993) Carbonylation of formaldehyde over ion exchange resin catalysts. Ind Eng Chem Res 32:253
Adamson K, Pearson P (2000) Hydrogen and methanol: a comparison of safety, economics, efficiencies and emissions. J Power Sources 86:548
Fu SS, Somorjai GA (1992) Roles of chemisorbed oxygen and zinc oxide islands on copper(110) surfaces for methanol decomposition. J Phys Chem 96:4542
Hsu WD, Ichihashi M, Kondow T, Sinnott SB (2007) Ab initio molecular dynamics study of methanol adsorption on copper clusters. J Phys Chem A 111:441
Spendelow JS, Goodpaster JD, Kenis PJA, Wieckowski A (2006) Methanol dehydrogenation and oxidation on Pt(111) in alkaline solutions. Langmuir 22:10457
Wang GC, Zhou YH, Morikawa Y, Nakamura J, Cai ZS, Zhao XZ (2005) Kinetic mechanism of methanol decomposition on Ni(111) surface: a theoretical study. J Phys Chem B 109:12431
Gong XQ, Selloni A, Vittadini A (2006) Density functional theory study of formic acid adsorption on anatase TiO2(001): geometries, energetics, and effects of coverage, hydration, and reconstruction. J Phys Chem B 110:2804
Branda MM, Collins SE, Castellani NJ, Baltanas MA, Bonivardi AL (2006) Methanol adsorption on the beta-Ga2O3 surface with oxygen vacancies: theoretical and experimental approach. J Phys Chem B 110:11847
El-Molla SA, Ismail SA, Ibrahim MM (2011) Effects of γ-irradiation and ageing on Surface and catalytic properties of nano-sized CuO/MgO system. J Mex Chem Soc 55(3):154–163
Ibrahim SM, Badawy AA, El-Shobaky GA, Mohamed HA (2014) Structural, surface and catalytic properties of pure and ZrO2-doped nanosized cobalt–manganese mixed oxides. Can J Chem Eng 92:676–684
Rossignol S, Kappenstein C (2001) Effect of doping elements on the thermal stability of transition alumina. Int J Inorg Mater 3:51
El-Molla SA (2006) Dehydrogenation and condensation in catalytic conversion of iso-propanol over CuO/MgO system doped with Li2O and ZrO2. Appl Catal A 298:103
El-Molla SA, El-Shobaky GA, Amin NH, Hammed MN, Sultan SN (2013) Catalytic properties of pure and K-doped CuO/MgO system towards 2-propanol conversion. J Mex Chem Soc 57(1):36
Diez VK, Apesteguia CR, Dicosimo JI (2000) Acid–base properties and active site requirements for elimination reactions on alkali-promoted MgO catalysts. Catal Today 63:53
El-Shobaky GA, Ghozza AM (2004) Effect of ZnO doping on surface and catalytic properties of NiO and Co3O4 solids. Mater Lett 58:699
Chen J, Qiao Y, Li Y (2008) Promoting effects of doping ZnO into coprecipitated Ni–Al2O3 catalyst on methane decomposition to hydrogen and carbon nanofibers. Appl Catal A 337:148
El-Shobaky GA, Radwan NRE, Radwan FM (2001) Investigation of solid–solid interactions between pure and Li2O-doped magnesium and ferric oxides. Thermochim Acta 380:27
Radwan NRE, El-Shobaky HG (2001) Solid–solid interactions between ferric and cobalt oxides as influenced by Al2O3-doping. Thermochim Acta 360:147
Zhou J-p, He H-c, Lin Y-h (2006) Effect of ZnO doping on the reaction between Co and Fe oxides. Mater Lett 60:1542
El-Shobaky HG, Ahmed AS, Radwan NRE (2006) Effect of γ-irradiation and ZnO-doping of CuO/TiO2 system on its catalytic activity in ethanol and isopropanol conversion. Colloids Surf A 274:138
El-Molla SA, Abdel-all SM, Ibrahim MM (2009) Influence of precursor of MgO and preparation conditions on the catalytic dehydrogenation of iso-propanol over CuO/MgO catalysts. J Alloys Compd 484:280
Klug HP, Alexander LE (1966) X-ray diffraction procedures for polycrystalline and amorphous materials. Wiley, New York, p 491
Dohiem MM, El-Boohy HA, Mokhater M, El-Shobaky GA (2001) Surface and catalytic properties of the γ-irradiated ZnO-treated Co3O4/Al2O3 system. Adsorpt Sci Technol 19:721
El-Shobaky GA, Amin NH, Deras NM, El-Molla SA (2001) Decomposition of H2O2 on pure and ZnO-treated Co3O4/Al2O3 solids. Adsorpt Sci Technol 19(1):45–58
Zaza P, Randall H, Dopper R, Renken A (1994) Dynamic kinetics of catalytic dehydrogenation of methanol to formaldehyde. Catal Today 20:325
Wojciechowska M, Haber J, Lomnicki S, Stoch J (1999) Structure and catalytic activity of double oxide system: Cu–Cr–O supported on MgF2. J Mol Catal A 141:155
Pepe F, Angeletti C, Rossi S, Jacono ML (1985) Catalytic behavior and surface chemistry of copper/alumina catalysts for isopropanol decomposition. J Catal 91:69
Henrich VE, Cox PA (1994) The surface science of metal oxides. Cambridge University Press, Cambridge
El-Shobaky GA, Mostafa AA (2003) Solid–solid interactions in Fe2O3/MgO system doped with aluminium and zinc oxides. Thermochim Acta 408:75
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
El-Molla, S.A., Ibrahim, S.M. & Ebrahim, M.M. Influence of ZnO doping and calcination temperature of nanosized CuO/MgO system on the dehydrogenation reactions of methanol. Int J Ind Chem 7, 223–229 (2016). https://doi.org/10.1007/s40090-016-0076-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-016-0076-x