Abstract
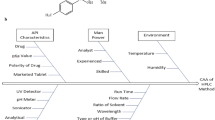
The aim of the proposed study was to develop a novel sensitive and rugged stability indicating HPLC method based on an approach of fractional factorial design (FFD) for the simultaneous of estimation of gliclazide and metformin hydrochloride without prior separation in tablets. Preliminary experiments were conducted to identify the critical attribute factors and using Taguchi screening method to assess risk management method process parameters. Later on FFD was applied to optimize the resolution as response between both drugs in short run time. Sixteen experimental runs were performed from grid search data using design of expert software v.7.0. Furthermore, the optimized method was then validated according to the ICH guidelines. The generated model using FFD for the resolution was found to be highly statistically significant (p < 0.05) and after analyzing the optimized zone within the design space, the final chromatographic conditions were selected as mobile phase 60:20:20:0.5, pH 7.5, flow rate 1.0 ml/min and column temperature 25 °C using analytical column C18 Nucleosil ODS (250 × 4.6 mm2 i.d, 5 µ) at 227 nm of detector wavelength. Validation of the developed method by QbD has manifested the specificity, accuracy and excellent linearity range and correlation values (R2) 0.997 and 0.996 for drugs. From the analysis it was measured that their LOD and LOQ values for the drugs were 0.035, 0.080 μg/ml and 0.40, 0.55 μg/ml. Therefore, a novel sensitive, accurate and stability indicating method was developed with high degree of practical utility of both drugs estimation in pharmaceutical dosage forms.





Similar content being viewed by others
References
Ahmad NR (2012) Spectrophotometric determination of metformin hydrochloride via oxidative coupling reaction with 1-naphthol in pharmaceutical and environmental water sample (NJC). Iraqi Natl J Chem 46:161–170
Akula A, Prajwala N, Sandhya M, Maheswara Rao U (2013) Development and validation of RP-HPLC method for simultaneous estimation of metformin hydrochloride and gliclazide in bulk and combined doasage form. Int J Pharm Pharm Sci 5:511–517
Beg S, Sharma G, Katare OP et al (2015) Development and validation of a stability-indicating liquid chromatographic method for estimating Olmesartan medoxomil using quality by design. J Chromatogr Sci. doi:10.1093/chromsci/bmu165
Belcher G, Lambert C, Edwards G et al (2012) Safety and tolerability of pioglitazone, metformin, and gliclazide in the treatment of type 2 diabetes. Diabetes Res Clin Pract 50:53–62. doi:10.1016/j.diabres.2005.02.011
Bhaskar R, Bhaskar R, Sagar MK, Saini V (2013) Multivariate chemometric assisted analysis of metformin hydrochloride, gliclazide and pioglitazone hydrochloride in bulk drug and dosage forms. Adv Pharm Bull 3:79–84. doi:10.5681/apb.2013.013
Dhabale PN, Seervi CR (2010) Simultaneous UV spectrophotometric method for estimation of gliclazide and metformine hydrochloride in tablet dosage form. Int J ChemTech Res 2:813–817
Fatema K, Rahman MZ, Haque T et al (2010) Development and validation of a simple method for simultaneous estimation of metformin hydrochloride and gliclazide in tablets by using reversed phase high performance liquid chromatography. Dhaka Univ J Pharm Sci 9:83–89. doi:10.3329/dujps.v9i2.7884
Foroutan SM, Zarghi A, Shafaati A, Khoddam A (2006) Application of monolithic column in quantification of gliclazide in human plasma by liquid chromatography. J Pharm Biomed Anal 42:513–516. doi:10.1016/j.jpba.2006.05.003
Garg NK, Sharma G, Singh B et al (2015) Quality by design (QbD)-based development and optimization of a simple, robust RP-HPLC method for the estimation of methotrexate. J Liq Chromatogr Relat Technol 38(17):1629–1637
Havele M (2011) Development and validation of a HPLC method for the determination of metformin hydrochloride, gliclazide and piogliglitazone hydrochloride in multicomponent. Webmedcentral 1:16
ICH (2005) International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use: ICH harmonised tripartite guideline. Valid Anal Proced Text Methodol Q2(R1) 1994:1–13
Jain D, Jain S, Jain D, Amin M (2008) Simultaneous estimation of metformin hydrochloride, pioglitazone hydrochloride, and glimepiride by RP-HPLC in tablet formulation. J Chromatogr Sci 46:501–504
Kolte BL, Raut BB, Deo AA et al (2004) Simultaneous high-performance liquid chromatographic determination of pioglitazone and metformin in pharmaceutical-dosage form. J Chromatogr Sci 42:27–31
Monks K, Molnar I, Rieger HJ et al (2012) Quality by design: multidimensional exploration of the design space in high performance liquid chromatography method development for better robustness before validation. J Chromatogr A 1232:218–230. doi:10.1016/j.chroma.2011.12.041
Nicholson G, Hall GM (2011) Diabetes mellitus: new drugs for a new epidemic. Br J Anaesth 107:65–73. doi:10.1093/bja/aer120
Park JH, Ryu YK, Lee HS, Lim HJ, Park JK, Lee YK, Jang MD, Suh JK, Carr PW (1999) Effect of triethylamine in the mobile phase on the retention properties of conventional polymeric and horizontally polymerized octadecylsilica in RPLC. J Chromatogr 49:635–642
Rao U, Nikalje AP (2011) Determination of gliclazide in a tablet dosage form in the presence of metformin hydrochloride by ion pair reversed phase liquid chromatographic technique. Afr J Pharm Pharmacol 5:1331–1337. doi:10.5897/AJPP10.106
Rao S, Rao N, Gajula R (2013) Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC–MS/MS and its application to a human pharmacokinetic study. J Pharm Anal 3:9–19. doi:10.1016/j.jpha.2012.09.002
Ravi BVV, Patnaik AK, Raul SK, Rao NN (2013) A RP-HPLC method development and validation for the estimation of gliclazide in bulk and pharmaceutical dosage forms. J Appl Pharm Sci 3:59–62. doi:10.7324/JAPS.2013.3410
Salim M, El-enany N, Belal F et al (2012) Simultaneous determination of sitagliptin and metformin in pharmaceutical preparations by capillary zone electrophoresis and its application to human plasma analysis. Anal Chem Insights. doi:10.4137/ACI.S9940
Sarkar A, Tiwari A, Bhasin PS, Mitra M (1982) Pharmacological and pharmaceutical profile of gliclazide: a review. J Appl Pharm Sci 01:11–19
Sena CM, Louro T, Matafome P et al (2009) Antioxidant and vascular effects of gliclazide in type 2 diabetic rats fed high-fat diet. Physiol Res 58:203–209
Setter S, Iltz J, Thams J, Campbell R (2003) Metformin hydrochloride in the treatment of type 2 diabetes mellitus: a clinical review with a focus on dual therapy. Clin Ther 25:2991–3026
Thummala VRR, Seshadri RK, Mohan Tharlapu SSJ et al (2014) Development and validation of a UPLC method by the QbD-approach for the estimation of rabeprazole and levosulpiride from capsules. Sci Pharm 82:307–326. doi:10.3797/scipharm.1310-17
Van Belle T (2011) Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 91:79–118. doi:10.1152/physrev.00003.2010
Xavier CM, Basavaiah K (2012) Implementation of quality by design for the development and validation of pioglitazone hydrochloride by RP-UPLC with application to formulated forms. doi:10.5402/2012/592849
Acknowledgments
Authors are thankful for financial support to this research project that was partially granted by Amar Shaheed Baba Ajit Singh Jujhar Singh Memorial College of Pharmacy, Ropar, India and Dr. Reddy Laboratories, H.P, India. Authors (Deepika Thakur, Amandeep Kaur, Suraj Sharma) declare that they do not have any conflict of interest and the paper has not been submitted elsewhere. This article does not contain any studies with human and animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thakur, D., Kaur, A. & Sharma, S. Application of QbD based approach in method development of RP-HPLC for simultaneous estimation of antidiabetic drugs in pharmaceutical dosage form. Journal of Pharmaceutical Investigation 47, 229–239 (2017). https://doi.org/10.1007/s40005-016-0256-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-016-0256-x