Abstract
Recombined low-fat dairy cream (RLFDC) primarily comprises anhydrous milk fat and milk protein. The primary advantage of RLFDC is that its ingredients can be easily stored and transported, whereas its most notable disadvantage is poor stability. The effects of glycerol monostearate (GMS) or sorbitan monooleate ethoxylate (Tween 80) on the physical properties and stability of RLFDC (20% fat and 2% protein) were investigated in this study. Physical properties were evaluated according to droplet size, surface protein concentration, ζ-potential, and apparent viscosity; stability was assessed according to creaming rate. Results showed that GMS and Tween 80 could significantly increase the creaming stability of RLFDC compared with a control sample. The creaming stability of RLFDC with GMS highly depended on ζ-potential and apparent viscosity, which contributed to electrostatic repulsion and intermolecular resistance. The effect of Tween 80 on the creaming stability of RLFDC was independent of the physical properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recombined dairy cream using anhydrous milk fat (AMF) and milk protein has become a common alternative to natural dairy cream. This alternative is preferred because the ingredients are anhydrate in nature, which means that they are raw materials during storage, transportation, and product development that can be easily adjusted according to desired portions and properties (Fredrick et al. 2013b; van Lent et al. 2008). Recombined low-fat dairy cream (RLFDC), for example, contains 20% AMF and is often used to make coffee creamer.
RLFDC is a thermodynamically unstable emulsion, which causes creaming, aggregation, coalescence, partial coalescence, phase inversion, and Ostwald ripening (Fredrick et al. 2010). Lowering the creaming rate is an effective way of improving the stability of RLFDC (Cheetangdee and Fukada 2012). As an oil-in-water emulsion, RLFDC is typically stabilized by adding proteins, surfactants, and thickeners. Proteins and low-molecular-weight surfactants (LMWSs) can coexist in the emulsion as emulsifiers (Pugnaloni et al. 2004; Zhao et al. 2013). LMWSs such as glycerol monostearate (GMS) and sorbitan monooleate ethoxylate (Tween 80) are widely applied in different types of oil-in-water emulsions. GMS is soluble in oil whereas Tween 80 is soluble in water, and both are typically different emulsifiers based on hydrophilic-lipophilic characterizations. Hence, GMS and Tween 80 are utilized in RLFDCs to improve emulsion stability.
Monoglycerides improve the long-term stability of food emulsions and facilitate the displacement of protein from oil surfaces (Sánchez and Patino 2004). GMS is a non-ionic ester of glycerol and stearic acid. As a saturated monoglyceride, GMS lends the emulsion higher orthokinetic stability than unsaturated monoglycerides. Fredrick et al. (2013a) proposed that saturated monoglycerides could prevent needle-like fat crystal shapes from deforming the interface for the chain crystallization of saturated monoglycerides behaving as a solid at the oil-in-water interface (Fredrick et al. 2010; Fredrick et al. 2013a). Moreover, nanocrystals of GMS absorbed on the oil surface could stabilize the emulsion (Munk et al. 2014). Compared with unsaturated monoglyceride that forms a dense fat network in the emulsion (Munk et al. 2014). saturated monoglyceride shows a lower partial coalescence rate, specifically in recombined dairy cream (Fredrick et al. 2013a). In addition, monoglyceride can incorporate with protein to form a dynamic monoglyceride-protein monolayer at an oil surface (Sánchez and Patino 2004).
Tween 80 is a non-ionic ester produced with sorbitan monooleate and ethoxylate and enhances the storage stability of emulsions through competitive displacement of surface proteins (Nikiforidis and Kiosseoglou 2011). Increasing the content of Tween 80 could reduce the creaming index of corn oil-in-water emulsions, but it has no significantly positive effect on viscosity (Koocheki and Kadkhodaee 2011). Tween 80 was reported to be more applicable to low-viscosity than high-viscosity emulsions (Züge et al. 2013).
Previous studies (mentioned above) on the contributions of GMS and Tween 80 to the physical characteristics (droplet size, apparent viscosity, and surface protein) of oil-in-water emulsions are abundant, but the relationship between the physical characteristics and creaming stability of RLFDC remains unclear.
The primary goal of this study was to investigate the relationship between the physical characteristics and stability of RLFDC. RLFDC was emulsified by various levels of GMS or Tween 80. The physical characteristics of RLFDC were evaluated according to droplet size, surface protein concentration, ζ-potential, and apparent viscosity. Stability was assessed according to creaming rate, which was measured using a centrifugal sedimentation method.
2 Materials and methods
2.1 Materials
Anhydrous milk fat was purchased from Fonterra Cooperative Group (Auckland, New Zealand), and sodium caseinate (SC; 92% of protein) was obtained from Arla Foods Ingredients Group (Leeds, UK). Milk protein concentrate (MPC; 72% protein) was kindly donated by Cezanne Co., Ltd. (Ningxia, China), whereas whey protein concentrate (WPC; 82% protein) was purchased from Hilmar ingredients (Dalhart, USA). GMS and Tween 80 were obtained from Danisco Co., Ltd. (Kunshan, China) and TCI (Shanghai, China) Development Co., Ltd., respectively. All other reagents were of analytical grade.
2.2 Preparation of recombined cream
The recombined cream consisted of 20% (w/w) AMF; 2% (w/w) protein from MPC, WPC, and SC mixtures at the protein ratio of 25:6:5; and various concentrations of GMS (0, 0.10, 0.20, and 0.40% w/w) or Tween 80 (0, 0.01, 0.02, 0.04, and 0.08% w/w).
MPC (1.98% w/w) was dissolved in deionized water and stirred with an overhead stirrer at 400 rpm and 50 °C for 4 h to complete the hydration process (RW 20.n, IKA Co., Ltd., Guangzhou, China). AMF was then heated to 80 °C to ensure complete melting, whereas WPC (0.40% w/w) and SC (0.37 %, w/w) were dispersed into the prepared MPC solutions and stirred at 50 °C for 15 min. Next, the surfactant (unheated), oil phase, and water phase were thoroughly mixed with a homogenizer XHF-D (Ningbo Scientz Biotechnology Co. Ltd., Ning Bo, China) at 8000 rpm and 50 °C for 4 min. The emulsion was immediately homogenized using a two-step laboratory-scale high-pressure homogenizer (China) with 15 Mpa pressure at the first stage and 4 Mpa at the second stage. After homogenization, the mixtures were sealed in bottles, sterilized at 115 °C for 15 min, and cooled to room temperature in a water bath. The samples underwent further measurements and analyses after storage at 4 °C for 1 day.
2.3 Determination of fat droplet size and distribution
A Malvern MasterSizer 3000 (Malvern Instruments Co., Ltd., Worcestershire, UK) was used to determine the droplet size distribution and average diameter of emulsion droplets. Each sample was measured in triplicate (Long et al. 2012). The refractive index, dispersed phase adsorption, and continuous phase refractive index were set to 1.52, 0.1, and 1.33, respectively. Surface weighted average diameter d 3,2 (μm) was calculated using the following equation:
where n i is the number of particles with the same diameter and d i is particle size.
2.4 Determination of surface protein concentration
Surface protein concentration of the emulsion samples was measured using methods from a previous study (Liu et al. 2012; Zhao et al. 2009) with some modifications. Each emulsion was centrifuged at 13,400×g for 60 min at 20 °C in a temperature-controlled centrifuge. The subnatant was carefully removed using a syringe and separated by filtration. Then, protein concentration of the subnatant was measured using the Kjeldahl nitrogen procedure. Specific surface area (SSA; m2.g) was measured using a MasterSizer 3000.
Surface protein concentration (Γ, mg.m-2) was calculated as follows:
where P 0 is the total protein in the emulsion (g), P s is the amount of protein measured in the subnatant (g), and M c is the weight of the creaming layer (g).
2.5 Determination of ζ-potential
The ζ-potential of RLFDC solutions was measured by laser Doppler velocimetry and dynamic light-scattering techniques using a Malvern Zetasizer Nano-ZS90 instrument (Malvern Instruments, Worcestershire, UK) at 25 °C (RLFDC is commonly stored at 25 °C except during preparation of RLFDC requiring 4 °C temperature). The solution samples were diluted with deionized water at a ratio of 1:100. One milliliter of each diluted sample was placed in a visibly clear disposable zeta cell (Model DTS 1060C, Malvern Instruments Co., Ltd., Worcestershire, UK) without any air or bubbles. Equilibrium time was 120 s before particle charge data was collected over 20 continuous readings. Measurements were carried out in triplicate.
2.6 Determination of apparent viscosity
The apparent viscosity of emulsion samples was determined using a Physica MCR 301 rheometer (Anton Paar Co., Ltd., Graz, Austria) with a CC27 rotor. The temperature was controlled at 25 °C using a Peltier System Viscotherm VT2 (Paar Physica), and rheological measurement data were analyzed using RheoPlus software (Anton Paar Co., Ltd., Graz, Austria). The dependence of apparent viscosity on shear rate was observed in the controlled rate mode. Shear rate was linearly increased from 5 to 89 s−1 for 240 s, and 36 points were obtained.
2.7 Determination of creaming stability
The physical stability of the emulsions was measured using a LUMiSizer (LUM GmbH, Germany), which employs centrifugal sedimentation to stimulate instability such as sedimentation, flocculation, or creaming. Near-infrared light illuminated the entire sample cell to measure the intensity of transmitted light while the emulsions were centrifuged. The instrument recorded changes in the transmitted light with time and position over the entire sample (Yuan et al. 2011). The obtained graph (integrated by computer) showed the percentage of light absorbance per time interval known as the “creaming rate.” The instrument parameters set for measurement were as follows: 3800 rpm, timeExp 3600 s, time interval 30 s, temperature 35 °C, and light factor 1.00. During the transport of RLFDC, 35 °C was an unpleasant temperature that occasionally occurred. Thus, 35 °C was applied to RLFDC during centrifugation to observe the creaming stability of the emulsion.
2.8 Statistical analysis
All measurements were carried out at least in duplicate, and the entire study was performed in two replicates. Statistical analysis was conducted using IBM SPSS 21 (IBM SPSS Inc., Chicago, IL, USA) for one-way ANOVA. Duncan multiple post hoc comparison test was employed to determine the significant differences among treatment means at P < 0.05.
3 Results and discussion
3.1 Droplet size and distribution
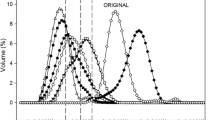
Droplet size and droplet size distribution of RLFDC with different levels of GMS or Tween 80 were observed (Figs. 1 and 2). The droplet size of RLFDC containing 0.10–0.40% GMS or 0.01–0.02% Tween 80 decreased significantly (P < 0.05) compared with the control sample, whose droplet size was not larger at 0.04–0.08% of Tween 80.
Droplet size distributions were all monomodal peaks, suggesting that fat droplets were mainly dispersed rather than aggregated. In addition, the observations by optical microscope (data not shown) showed that the oil droplets were mainly dispersed in the RLFDC emulsions. Surfactant molecules greatly reduce interfacial tension by absorbing into oil–water interfaces, thereby facilitating further disruption of emulsion droplets during homogenization. High surfactant level also enhances the surfactant’s ability to absorb into the interface and decreases the time needed to cover the interface (Koocheki and Kadkhodaee 2011). Furthermore, non-ionic surfactants create increased surface area by forming smaller droplets than protein during emulsification (Courthaudon et al. 1991). Hence, droplet size decreased as GMS increased. In Tween 80 RLFDC, droplet size also decreased at the 0.01% level and remained constant until the 0.02% Tween 80 level, as the surfactants adsorbed onto the interface were not enough to decrease the droplet size. The increased droplet size at 0.04% Tween 80 suggested the balance between Tween 80 and protein on the oil droplet surface, where the combined emulsifying effect of surfactant and protein was poor. A negative synergism was also reported by Dickinson et al. (1999).
3.2 Surface protein concentration
The adsorbed layers of proteins and surfactants were assessed by measuring the degrees of protein adsorption. The effects of GMS or Tween 80 levels on the surface protein concentration of RLFDC were examined (Figs. 3a and 4a). Surface protein concentration was gradually and significantly (P < 0.05) reduced to levels lower than that of the control sample as the GMS level (0.05–0.40%) increased. The surface protein concentration of RLFDC with 0.01, 0.02, and 0.08% Tween 80 showed no significant changes but was still significantly lower than that of the control sample. Surface protein concentration with 0.04% Tween 80 exhibited no significant difference with the control sample.
Fat droplet stabilization is influenced considerably by surface protein concentration (Kanno et al. 1991), and surfactant-displacing proteins from oil surfaces reduce surface protein concentration (Zhao et al. 2013). A decline in surface protein concentration in GMS (0–0.40%) was observed along with the increase of surfactant. The surface protein concentration at 0.01, 0.02, and 0.08% Tween 80 was significantly lower than that of the control sample. According to Eq. (2), the increase of surface protein concentration at 0.04% Tween 80 was due to the increase of droplet size which substantially reduced the SSA of oil droplets, despite the decrease of surface protein mass (data not shown).
3.3 ζ-potential
Fat droplet surfaces were negatively charged at pH 6.8 in RLFDC because of the proteins. Figure 3b illustrates that the addition of 0.05% GMS led to a significant decrease in ζ-potential (from ζ = −40.17 mV without monoglyceride to ζ = −36.62 mV with 0.05% monoglyceride). When RLFDC was emulsified by higher levels of GMS, ζ-potential increased gradually. The ζ-potential decreased significantly (P < 0.05) to −34.25 mV at 0.01% Tween 80 and decreased to −31.83 mV at 0.02% Tween 80 (Fig. 4b). At 0.04% Tween 80, an increase in ζ-potential was observed compared with the 0.20% level, whereas the ζ-potential at 0.08% was slightly reduced without significant difference with the 0.04%.
Assessing changes in the surface charge density of fat droplets is an efficient way of quantifying the electrostatic repulsive force between droplets. The “orogenic” mechanism functions as surfactant molecules nucleate at defects in the protein interface during the first stage of displacement (Mackie et al. 1999, 2000). Surfactants adsorbed into the surface protein demonstrated the effects of shielded ζ-potential. As a result, 0–0.05% GMS or 0–0.01% Tween 80 decreased the ζ-potential significantly. At these surfactant concentrations, surfactants spread across and covered the protein layers as the surfactant-fat domains were not enough to accommodate GMS or Tween 80, resulting in a significant decline of ζ-potential. Furthermore, as GMS was soluble in oil and Tween 80 was soluble in water, GMS was more effectively adsorbed into the protein close to the surfactant-fat domains compared with Tween 80. This difference between GMS and Tween 80 allowed the former to successfully concentrate on the surfactant-fat domains, particularly at high levels of surfactant concentration, which resulted in the reduction of shielding effect. Therefore, the ζ-potential of RLFDC gradually increased as GMS increased (0.05–0.40%), whereas no significant increase of ζ-potential was observed as Tween 80 increased (0.01–0.08%). At 0.02% Tween 80, the ζ-potential was decreased significantly, which probably resulted from the overcoverage of Tween 80 on the protein layer of oil droplet.
3.4 Apparent viscosity
Curves that represent apparent viscosity versus shear rate according to different concentrations of GMS and Tween 80 are shown in Fig. 5. The RLFDC showed Newtonian fluid characteristics at 0, 0.05, and 0.10% GMS and exhibited shear rate dependency and shear-thinning behavior at 0.20 and 0.40% GMS. Apparent viscosity steadily increased as GMS concentration increased (0–0.20%). However, apparent viscosity showed no difference during the 0.20–0.40% GMS when the shear rate was lower than 22 s−1. When the shear rate was higher than 22 s−1, the apparent viscosity at 0.40% GMS became significantly lower than that at 0.20% GMS. Tween 80 RLFDC showed an almost Newtonian flow behavior. The apparent viscosity decreased between 0 and 0.01% Tween 80 and then increased at 0.01–0.02%. With 0.02–0.08% Tween 80, the apparent viscosity of RLFDC decreased. Except at 0.02%, the apparent viscosity exhibited similar values at other levels.
Reduction of droplet size and increase in droplet concentration in the oil-in-water emulsions are associated with increased apparent viscosity (Chanamai and McClements 2000). In addition, the droplet association for the monoglyceride at the interface of the neighboring oil can increase the apparent viscosity of emulsions (Marangoni et al. 2007; Mao et al. 2014). Because the oil droplet size of GMS decreased, the oil droplet concentration increased when oil content was constant. Hence, the apparent viscosity of RLFDC during 0–0.20% GMS increased. However, the viscosity between 0.20 and 0.40% GMS indicated a strong droplet association at 0.20% GMS. The step of surfactant displacing protein is cover on it first. The GMS coverage on the oil surface at 0.20% was probably larger than that at 0.40%. The first emergence of the non-Newtonian behavior at 0.20% GMS confirmed the strong association.
RLFDC with Tween 80 showed nearly Newtonian properties with very low viscosity, which has been detected in emulsions containing 1% Tween 80 and 20% oil (Mao et al. 2014). At 0.01% Tween 80, the apparent viscosity decreased compared with the control sample, which is not consistent with the findings of Marangoni et al. (2007). This result suggested that the interface of oil droplets in 0.01% Tween 80 was not rigid enough compared with that in GMS. At 0.01–0.02% Tween 80, the droplet size and surface protein concentration were not significantly changed as the surfactant level increased. This behavior indicated that Tween 80 mainly covered the protein layer of oil surface and this coverage was large, which was further proven by the decreased ζ-potential. The dangling tail of adsorbed Tween 80 molecules stuck onto other droplet surfaces and droplet association increase apparent viscosity (Dickinson et al. 1999; Mao et al. 2014). Consequently, apparent viscosity increased significantly in 0.02% Tween 80 as a result of associations between droplets during shearing. At 0.02–0.04%, Tween 80 was more concentrated on the surfactant-fat domains and its coverage on the oil surface decreased, which in turn reduced the apparent viscosity compared with 0.02% Tween 80. The decreasing apparent viscosity from 0.04 to 0.08% Tween 80 was probably associated with the non-rigid oil interface as that of 0.01% Tween 80.
3.5 Creaming stability
To evaluate RLFDC stability, the creaming rate of each sample was measured using an accelerated test. Creaming rate decreased slightly at 0.05% GMS and was reduced significantly at 0.10% GMS compared with the control sample. It remained steady at 0.20% and significantly decreased in absence of 0.40% GMS compared with the 0.10% (Fig. 6a). In the Tween 80 samples, the creaming rate significantly decreased compared with the emulsion without emulsifiers. The most significant decrease in creaming rate was observed at 0.04% Tween 80, whereas the creaming rate at 0.01% Tween 80 showed no significant difference with 0.02 and 0.08% Tween 80.
Lower creaming rate indicates better colloidal stability (Cheetangdee and Fukada 2012). After the addition of GMS, the RLFDC gradually became more stable against creaming, which indicates that GMS effectively prevented the majority of droplets from creaming during centrifugation. Electrostatic repulsion and interaction repulsion forces between droplets have been shown to enhance stabilizing effects (Jourdain et al. 2008). As shown in Figs. 3b and 5a, ζ-potential and viscosity increased as GMS concentration increased. Higher ζ-potential and viscosity enhanced RLFDC stability at 0.40% GMS.
Tween 80 RLFDC samples exhibited better creaming stability than the control sample. The viscosity of RLFDC was higher at 0.02% Tween 80, but the droplets were destabilized by strong associations that tend to aggregate during shear behavior, as confirmed by high creaming rate at 0.02% Tween 80. The lowest creaming rate was observed at 0.04% concentration, without any obvious advantages of ζ-potential and viscosity. Nevertheless, although ζ-potential decreased, all RLFDC samples with Tween 80 were more stable than the control sample. These results suggested that instead of apparent viscosity and electrostatic repulsion, the synthetic emulsifying effect of Tween 80 and proteins on oil surfaces may play a crucial role in the stabilizing effect of emulsions in RLFDC with Tween 80.
4 Conclusion
The results of this study indicated that increased ζ-potential and apparent viscosity reduced the creaming rate of RLFDC with 0.40% GMS, where the electrostatic repulsion and intermolecular resistance contributed to better emulsion stability. The RLFDC at 0.04% of Tween 80 had better stability than other concentration. (Although the creaming rate at 0.04 and 0.08% Tween 80 showed no significant difference, the addition of Tween 80 would be favored by practical manufacturers.) In the current study, the physical properties of Tween 80 RLFDC did not play a positive role in increasing the creaming stability of emulsion. However, further research needs to be carried out to evaluate the changes in physical properties and stability of RLFDC during storage.
References
Chanamai R, McClements DJ (2000) Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surf A 172(1–3):79–86
Cheetangdee N, Fukada K (2012) Protein stabilized oil-in-water emulsions modified by uniformity of size by premix membrane extrusion and their colloidal stability. Colloids Surf A 403:54–61
Courthaudon J-L, Dickinson E, Dalgleish DG (1991) Competitive adsorption of β-casein and nonionic surfactants in oil-in-water emulsions. J Colloid Interface Sci 145:390–395
Dickinson E, Ritzoulis C, Povey MJW (1999) Stability of emulsions containing both sodium caseinate and Tween 20. J Colloid Interface Sci 212(2):466–473
Fredrick E, Walstra P, Dewettinck K (2010) Factors governing partial coalescence in oil-in-water emulsions. Adv Colloid Interf Sci 153(1–2):30–42
Fredrick E, Heyman B, Moens K, Fischer S, Verwijlen T, Moldenaers P, Van der Meeren P, Dewettinck K (2013a) Monoacylglycerols in dairy recombined cream: II. The effect on partial coalescence and whipping properties. Food Res Int 51(2):936–945
Fredrick E, Moens K, Heyman B, Fischer S, Van der Meeren P, Dewettinck K (2013b) Monoacylglycerols in dairy recombined cream: I. The effect on milk fat crystallization. Food Res Int 51(2):892–898
Jourdain L, Leser ME, Schmitt C, Michel M, Dickinson E (2008) Stability of emulsions containing sodium caseinate and dextran sulfate: relationship to complexation in solution. Food Hydrocoll 22(4):647–659
Kanno C, Shimomura Y, Takano E (1991) Physicochemical properties of milk fat emulsions stabilized with bovine milk fat globule membrane. J Food Sci 56:1219–1223
Koocheki A, Kadkhodaee R (2011) Effect of Alyssum homolocarpum seed gum, Tween 80 and NaCl on droplets characteristics, flow properties and physical stability of ultrasonically prepared corn oil-in-water emulsions. Food Hydrocoll 25(5):1149–1157
Liu L, Zhao Q, Liu T, Kong J, Long Z, Zhao M (2012) Sodium caseinate/carboxymethylcellulose interactions at oil–water interface: relationship to emulsion stability. Food Chem 132(4):1822–1829
Long Z, Zhao Q, Liu T, Kuang W, Xu J, Zhao M (2012) Role and properties of guar gum in sodium caseinate solution and sodium caseinate stabilized emulsion. Food Res Int 49(1):545–552
Mackie AR, Gunning AP, Wilde PJ, Morris VJ (1999) Orogenic displacement of protein from the air/water interface by competitive adsorption. J Colloid Interface Sci 210(1):157–166
Mackie AR, Gunning AP, Wilde PJ, Morris VJ (2000) Orogenic displacement of protein from the oil/water interface. Langmuir 16(5):2242–2247
Mao L, Calligaris S, Barba L, Miao S (2014) Monoglyceride self-assembled structure in O/W emulsion: formation, characterization and its effect on emulsion properties. Food Res Int 58:81–88
Marangoni AG, Idziak SHJ, Vega C, Batte H, Ollivon M, Jantzi PS, Rush JWE (2007) Encapsulation-stucturing of edible oil attenuates acute elevation of blood lipids and insulin in humans. Soft Matter 3(2):183–187
Munk MB, Larsen FH, van den Berg FWJ, Knudsen JC, Andersen ML (2014) Competitive displacement of sodium caseinate by low-molecular weight emulsifiers and the effects on emulsion texture and rheology. Langmuir 30(29):8687–8696
Nikiforidis CV, Kiosseoglou V (2011) Competitive displacement of oil body surface proteins by Tween 80—effect on physical stability. Food Hydrocoll 25(5):1063–1068
Pugnaloni LA, Dickinson E, Ettelaie R, Mackie AR, Wilde PJ (2004) Competitive adsorption of proteins and low-molecular-weight surfactants: computer simulation and microscopic imaging. Adv Coll Interf Sci 107(1):27–49
Sánchez CC, Patino JMR (2004) Surface shear rheology of WPI-monoglyceride mixed films spread at the air-water interface. Coll Surf B 36(1):57–69
van Lent K, Le CT, Vanlerberghe B, Van der Meeren P (2008) Effect of formulation on the emulsion and whipping properties of recombined dairy cream. Int Dairy J 18(10–11):1003–1010
Yuan F, Xu D, Qi X, Zhao J, Gao Y (2011) Impact of high hydrostatic pressure on the emulsifying properties of whey protein isolate–chitosan mixtures. Food Bioprocess Technol 6(4):1024–1031
Zhao Q, Zhao M, Li J, Yand B, Su G, Cui C, Jiang Y (2009) Effect of hydroxypropyl methylcellulose on the textural and whipping properties of whipped cream. Food Hydrocoll 23(8):2168–2173
Zhao Q, Kuang W, Long Z, Fang M, Liu D, Yang B, Zhao M (2013) Effect of sorbitan monostearate on the physical characteristics and whipping properties of whipped cream. Food Chem 141(3):1834–1840
Züge LCB, Haminiuk CWI, Maciel GM, Silveira JLM, Scheer AP (2013) Catastrophic inversion and rheological behavior in soy lecithin and Tween 80 based food emulsions. J Food Eng 116(1):72–77
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31471689), the Earmarked Fund for Modern Agro-Industry Technology Research System (CARS-37), the Planning Subject of “the twelth five-year-plan” in National Science and Technology for the Rural Development in China (2013BAD18B05-02, 2013BAD18B12-04, and 2013BAD18B12-05), Special Fund for Agro-Scientific Research in the Public Interest (201303085), and Synergetic Innovation Center of Food Safety and Nutrition.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Wu, S., Wang, G., Lu, Z. et al. Effects of glycerol monostearate and Tween 80 on the physical properties and stability of recombined low-fat dairy cream. Dairy Sci. & Technol. 96, 377–390 (2016). https://doi.org/10.1007/s13594-015-0274-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13594-015-0274-x