Abstract
Viral infections have been implicated as a cause of complex seizures in children. The pathogenic differences in complex seizures due to influenza A(H1N1)pdm09 or rotavirus gastroenteritis remain unclear. This study analyzed the gene expression profiles in the peripheral whole blood from pediatric patients with complex seizures due to influenza A(H1N1)pdm09 or rotavirus gastroenteritis. The gene expression profiles of ten patients (five with seizures and five without) with influenza A(H1N1)pdm09 and six patients (three with seizures and three without) with rotavirus gastroenteritis were examined. Gene expression profiles in the whole blood were different in complex seizures due to influenza A(H1N1)pdm09 or rotavirus gastroenteritis. Transcripts related to the immune response were significantly differentially expressed in complex seizures with influenza A(H1N1)pdm09, and transcripts related to the stress response were significantly differentially expressed in complex seizures with rotavirus gastroenteritis. Pathway analysis showed that the mitogen-activated protein kinases in the T cell receptor signaling pathway were activated in complex seizures due to influenza A(H1N1)pdm09. Dysregulation of the genes related to immune response or stress response could contribute to the pathogenic differences of the complex seizures due to influenza A(H1N1)pdm09 or rotavirus gastroenteritis.
Similar content being viewed by others
Introduction
Seizures are the most frequently reported neurological complication, and most of these manifest as single uncomplicated febrile convulsions in young patients. However, some patients exhibit complex seizures, such as partial, prolonged, and repeated seizures. Viral infections have been implicated as a cause of seizures (Millichap and Millichap 2006), and certain viruses, such as human herpes virus type 6, influenza virus, rotavirus, enterovirus, and adenovirus, are more frequently involved than others in pediatric seizures. Each viral infection appears to have characteristics in terms of the frequency, appearance, and outcome of seizures (Hoshino et al. 2012; Kolski et al. 1998; Lloyd et al. 2010; Newland et al. 2007).
In 2009, influenza A(H1N1)pdm09 virus spread globally to become a pandemic infection. This virus caused severe illness, with some patients developing severe acute respiratory distress syndrome (Davies et al. 2009). In addition, neurological manifestations were also reported in many cases worldwide, and seizures were frequently identified as neurological complications (Frobert et al. 2011; Glaser et al. 2012; Kedia et al. 2011; Surana et al. 2011). The majority of cases were simple febrile seizures with no sequelae, but some cases with high morbidity and mortality were described to progress rapidly with a high fever, alteration of cognition, and prolonged complex seizures, which was called influenza-associated encephalopathy (Morishima et al. 2002; Kawashima et al. 2012). The underlying pathophysiological mechanism of the influenza-associated encephalopathy is thought to be production of various inflammatory cytokines (Ichiyama et al. 2003; Ito et al. 2011).
Rotavirus is the most common cause of severe gastroenteritis diarrhea in young children, causing severe dehydration and electrolyte imbalances. Rotavirus gastroenteritis is accompanied by neurological manifestations, such as seizures, aseptic meningitis, and encephalitis. Afebrile seizures in the absence of dehydration, electrolyte imbalance, or hypoglycemia have also been described (DiFazio et al. 2007; Lynch et al. 2001). Despite having a relatively good prognosis, seizures often occur in clusters and are difficult to treat effectively. To date, the mechanism of central nervous system (CNS) involvement due to rotavirus gastroenteritis is unclear.
To reveal the pathogenic differences in the complex seizures of pediatric patients with influenza A(H1N1) pdm09 or rotavirus gastroenteritis, we analyzed the gene expression profiles in isolated peripheral whole blood cells using an oligonucleotide microarray.
Methods
Clinical characteristics of the patient group
Ten patients with influenza A(H1N1)pdm09 and six with rotavirus gastroenteritis were recruited between January 2009 and January 2010. Among the ten patients with influenza A(H1N1)pdm09, five were hospitalized for complex seizures (Flu-Seizure group; age 4.5–9.7 years, one boy and four girls) and five were uncomplicated (Flu-Control group; age 8.7–11.2 years, four boys and one girl). Among the six patients with rotavirus gastroenteritis, three were hospitalized for complex seizures (Rota-Seizure group; age 0.8–1.7 years, one boy and two girls) and three were uncomplicated (Rota-Control group; age 0.8–2.9 years, three boys). Influenza A(H1N1)pdm09 infection was confirmed using a commercial rapid diagnostic kit that can identify influenza A and B viruses using an immunochromatographic method and by A(H1N1)pdm09-specific polymerase chain reaction (PCR). Rotavirus antigen was confirmed in stool specimens with a commercial rapid diagnostic kit that can identify type A rotavirus. Complex seizures were defined as prolonged seizures of >15 min duration, multiple seizures within 24 h, or prolonged postictal impairment of consciousness for >30 min. None of the patients had underlying disease or a family history of the disease, and none had been vaccinated against influenza A(H1N1)pdm09 or rotavirus. All of the Flu-Seizure patients showed febrile complex seizures, and all of the Rota-Seizure patients showed afebrile complex seizures. Rotavirus RNA was not detected in any of the blood samples in the Rota-Seizure group. All patients, including hospitalized patients, recovered completely. Blood tests in the acute phase revealed that leukocytosis without highly increased C-reactive protein, which was supposed to result from seizure stress, was observed in three cases in the Flu-Seizure group and one case in the Rota-Seizure group. Case 1 in the Rota-Control group had hyponatremia due to hypotonic dehydration, and case 3 in the Rota-Control group had hypernatremia and increased blood urea nitrogen due to hypertonic dehydration. No electrolyte imbalance was detected in the Rota-Seizure group. No abnormalities of other tests including blood sugar and ammonia were observed in any of the patients (Table 1).
The study was conducted in accordance with the appropriate clinical and experimental ethical guidelines and was approved by the Ethics Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Science. Informed consent was obtained from all patients and/or their parents for their participation in this study.
Whole blood collection and RNA isolation
Blood samples were collected in PAXgene Blood RNA tubes (Qiagen, Valencia, CA, USA) from patients in the acute and recovery phases. For patients with complex seizures, the blood samples were collected on the day of admission (acute phase: Flu-Seizure, 1–2 days from disease onset; Rota-Seizure, 2–4 days from disease onset) and on the day of discharge (recovery phase: Flu-Seizure, 4–6 days from disease onset; Rota-Seizure, 7–9 days from disease onset). For the control group, samples were collected at the initial hospital visit (acute phase: Flu-Control, 1–3 days from disease onset; Rota-Control, 2–4 days from disease onset) and ~1 week later (recovery phase: Flu-Control, 6–9 days from disease onset; Rota-Control, 9–11 days from disease onset). After sample collection, the PAXgene tubes were incubated at room temperature for 2 h and then stored at −80 °C until RNA extraction. Total RNA was isolated using the PAXgene Blood RNA Kit (Qiagen). The RNA was treated with DNase for the removal of contaminating genomic DNA. The quality of the RNA was verified using a RNA 6000 Nano LabChip on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA), and the quantity of RNA was determined by a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The total RNA samples from the blood had RNA integrity number values >8. The ratio of OD260 and OD280 was calculated for all samples, and was between 1.8 and 2.1.
Microarray procedure
Synthesis of cDNA, cRNA labeling, and hybridization were performed according to established protocols (Affymetrix, Santa Clara, CA, USA). One microgram of total RNA sample was converted to double-stranded cDNA with a T7-oligo(dT) primer, and cDNA was transcribed in vitro to biotinylated cRNA. Fragmented biotin-labeled cRNA was hybridized to Affymetrix Human Genome U133 Plus 2.0 arrays in a hybridization oven for 16 h. After washing, the chips were stained with streptavidin–phycoerythrin and scanned. The raw and processed data were deposited in the National Center for Biotechnology Information Gene Expression Omnibus database (accession no. GSE50628).
Microarray data analysis
The Affymetrix Microarray Suite 5.0 Statistical Algorithm was used to calculate the signal intensity and the detection calls for each gene. The low expression probes were excluded, and the probes classified with absent detection calls in all samples were also excluded. Probe datasets were normalized through the following steps using the Subio Platform software (Subio, Nagoya, Japan): shifting of low signals <1.0 to 1.0, log-based transformation, and global normalization to the 75th percentile. To eliminate any individual differences, the ratio of the signal of the acute phase to that of the recovery phase (acute/recovery: processed signal) in each patient was calculated for further statistical analysis (Yamashita et al. 2013). To analyze the distribution of the gene expression patterns, principal component analysis (PCA) and hierarchical clustering analysis (HCA) were performed using the differentially expressed probe sets between the four groups identified by one-way analysis of variance (ANOVA) (p < 0.05). Differentially expressed genes between the seizure and control groups were defined by the unpaired Student’s t test with values of p < 0.05 and a fold change <0.5 or >2. Differentially expressed genes were subjected to gene ontology (GO) analysis using the Database for Annotation, Visualization and Integrated Discovery (http://david.abcc.ncifcrf.gov/). A pathway-specific analysis was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway database.
Quantitative reverse transcriptase PCR
Quantitative reverse transcriptase PCR (qRT-PCR) was used to assess and confirm expression levels of selected genes. Total RNA was reverse transcribed using an Omniscript kit (Qiagen) to generate cDNA, which was quantified using Power SYBR Green (Applied Biosystems, Foster City, CA, USA) on the Applied Biosystems 7500 Real-Time PCR system. The PCR conditions comprised 10 min at 95 °C and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Levels of gene expression were calculated using the comparative cycle threshold method of relative quantification with the GAPDH gene as an endogenous control. Primer sequences are listed in Supplementary material 1.
Results
Multidimensional scaling and clustering analysis of microarray data
To analyze the distribution of the gene expression patterns of the four groups, we first performed unsupervised HCA with 4250 significantly differentially expressed probe sets after one-way ANOVA across these groups. The HCA identified four dominant expression clusters that corresponded to samples belonging to each group, except for the sample from Rota-Control 1. We also performed a multidimensional scaling to display the position of each sample in a three-dimensional space using the PCA method. Each patient appeared to be distributed into one of four groups, suggesting that the global gene expression patterns were different between each of the groups (Fig. 1).
a HCA of gene expression in whole blood from each patient. The dendrogram originated from the clustering of values with Euclidean distances. The distance of the cluster branches was proportional to the differences in gene expression profiles between the patients. The color indicates the ratio of the signal of the acute phase to that of the recovery phase (acute/recovery: processed signal) in each patient. Red and blue indicate the relative upregulated and downregulated processed signals, respectively. b Multidimensional scaling analysis of the gene expression in the whole blood samples from each patient. Samples are displayed in a three-dimensional space consisting of the first three principal components (PC1, PC2, and PC3)
Identification of differentially expressed transcripts in the seizure groups
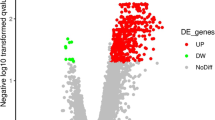
A total of 1,111 transcripts were upregulated and 488 were downregulated in the Flu-Seizure group compared with the Flu-Control group. In contrast, 558 transcripts were upregulated and 449 were downregulated in the Rota-Seizure group compared with the Rota-Control group. The top 100 upregulated and top 100 downregulated genes in the Flu-Seizure or Rota-Seizure group ranked by the fold changes are listed in Supplementary material 2 and 3. The Venn diagram shows 11 transcripts that were upregulated and 8 that were downregulated in the Flu-Seizure and Rota-Seizure groups (Fig. 2).
a Volcano plots of the comparison between the complex seizure and control groups, at p < 0.05 and a >2- or <0.5-fold-change cutoff (represented by lines). The x-axis represents the log2 of the fold change and the y-axis represents the p value. Each transcript was represented by a single point, and dark areas indicate significant changes in gene expression. b Venn diagrams of the up- or downregulated transcripts in the complex seizure group associated with influenza A(H1N1)pdm09 or rotavirus gastroenteritis. The numbers of transcripts with significant (p < 0.05, >2- or <0.5-fold change) changes in expression are listed
Functional analysis of the differentially expressed genes in the seizure groups
GO analysis revealed 150 significantly enriched GO terms in biological processes for the Flu-Seizure group and 33 for the Rota-Seizure group. Figure 3 shows the top 15 enriched GO term categories for the each group. The Flu-Seizure and Rota-Seizure groups showed common GO terms related to the positive regulation of apoptosis, cell death, and programmed cell death. Enriched GO terms related to the immune response were found in the Flu-Seizure group (p = 0.004), but not in the Rota-Seizure group. Of 45 significantly differentially expressed transcripts related to the immune response in the Flu-Seizure group, the transcripts of the interleukin-1 receptor (IL-1R)1/Toll-like receptor (TLR) superfamily (IL-18R1, IL-1R1, IL-1R2, IL-1R accessory protein, TLR4, and TLR8) were increased (Table 2). In contrast, the most significant enriched GO term in the Rota-Seizure group besides apoptosis-related GO terms was the cellular response to stress (p = 0.004), and this GO term was not significantly enriched in the Flu-Seizure group. Of the GO term related to cellular response to stress, eight genes were up- or downregulated in the Rota-Seizure group (Table 3).
Nine KEGG pathways were significantly enriched in the transcripts associated with the Flu-Seizure group, and two were significantly enriched in the transcripts associated with the Rota-Seizure group (Fig. 4). Among these pathways, the Fcγ-receptor-mediated phagocytosis pathway showed the most significant differences in the Flu-Seizure and Rota-Seizure groups (Flu-Seizure, p = 0.004; Rota-Seizure, p = 0.005). The T cell receptor (TCR) signaling pathway was significantly involved in the Flu-Seizure group (p = 0.013), but not the Rota-Seizure group. Of the transcripts that were involved in the TCR signaling pathway, mitogen-activated protein kinase (MAPK) family members [p38 MAPK and extracellular signal-regulated kinase (ERK)] were upregulated significantly in the Flu-Seizure group (Fig. 5).
Validation of microarray
qRT-PCR revealed that the processed signals of IL-1R/TLR superfamily were upregulated significantly in the complex seizures due to influenza A(H1N1)pdm09, and that the processed signals of four genes related to cellular response to stress (SOD2, OXR1 and HIGD1A) were up- or downregulated significantly in the Rota-Seizure group (Table 4).
Discussion
We analyzed the gene expression profiles associated with complex seizures in pediatric patients with influenza A(H1N1)pdm09 or rotavirus gastroenteritis, using an oligonucleotide microarray. Neither influenza A(H1N1)pdm09 virus nor rotavirus were detected in the brain of patients with complex seizures or encephalopathy in most of previous studies. However, complex seizures were present as clinical neurological manifestations in the patients infected with these viruses as well as other viruses such as human herpes simplex virus, enterovirus, and adenovirus, which can invade the CNS directly. We think it is important to reveal how these viruses can cause complex seizures despite local infection such as bronchiolitis or gastroenteritis. It is informative to analyze the gene expression of the brain in patients with encephalitis, but we analyzed the gene expression using peripheral blood to compare the complex seizures caused by respiratory and enteric viruses.
By using a commercially available method to stabilize RNA immediately in the blood, we preserved the immunological alterations that were present in vivo, with minimal interference from artifacts that arose from blood handling, storage, and separation. The disadvantage of this approach was that once the whole blood was stabilized, it was not possible to separate subsequently the different cell populations. However, this approach makes sample collection practical in a busy outpatient clinical setting, and data retention is maximized by the immediate stabilization of the RNA at the bedside.
In our study, PCA and HCA showed the apparent cluster of each of the groups. We also even observed different clusters between the Flu-Control and Rota-Control groups. We suppose that this result was caused by the difference in host immune response in the blood against influenza A(H1N1)pdm09 virus or rotavirus. Previous studies have demonstrated that differential expression of several genes is involved in proinflammatory cytokine formation, activation of lymphocytes, or antiviral response in the blood cells from children with influenza or rotavirus gastroenteritis (Wang et al. 2007; Xu et al. 2006). The gene expression profile of influenza patients without seizures shows that immune response genes, such as interferon (IFN)-regulated genes, were strongly upregulated in the acute phase (Kawada et al. 2006). We considered that the difference in gene expression between the Flu-Control and Rota-Control groups needed to be eliminated as background, to discuss the proper differentially expressed genes that contributed to progression of complex seizures in each viral infection. By selecting the differentially expressed genes by comparison between each seizure group and the control group, we were able to observe that only a few differentially expressed transcripts overlapped in complex seizure patients with both influenza A(H1N1)pdm09 and rotavirus gastroenteritis. These results strongly indicate that the genes related to progression to complex seizures differ greatly between influenza A(H1N1)pdm09 and rotavirus gastroenteritis.
Our investigations identified a significant dysregulation of IL-1R/TLR signaling in patients with complex seizures due to influenza A(H1N1)pdm09. TLRs play a crucial role in the early host defense against invading pathogens (Kawai and Akira 2010). TLR7/8 can recognize single-stranded RNA (ssRNA) viruses, such as influenza virus (Heil et al. 2004). TLR8 is predominantly expressed in the lungs and peripheral blood leukocytes, especially monocytes (Bekeredjian-Ding et al. 2006; Hart et al. 2005; Peng et al. 2005). The interaction between the ssRNA and TLR7/8 triggers the activation of nuclear factor-κB and other transcription factors and the production of proinflammatory cytokines and chemokines (Qin et al. 2006). In contrast, TLR4 can recognize the lipopolysaccharide in most Gram-negative bacteria (Hoshino et al. 1999). Recent studies have reported that TLR4 knockout mice are highly refractory to influenza-induced lethality, and that therapeutic antagonism of TLR4 signaling protects against influenza-induced acute lung injury (Abdul-Careem et al. 2011; Shirey et al. 2013). The increase in TLR4 transcripts in complex seizure patients due to influenza A(H1N1)pdm09 could play an important role in host defense against influenza A(H1N1)pdm09, through activation of innate immunity, but the mechanism by which TLR4 protects against infection with influenza A(H1N1)pdm09 is not known at present.
During influenza virus infection, IL-1β and IL-18 are mainly produced by macrophages and dendritic cells as part of the innate immune response. IL-1β can bind IL-1R, and IL-1R1 is primarily responsible for transmitting the inflammatory effects of IL-1. IL-1R1 knockout mice infected with influenza A virus show significantly impaired neutrophil recruitment, diminished T cell responses, impaired IgM responses, and increased incidence of virus-induced fatality (Schmitz et al. 2005). In contrast, IL-18R1 specifically binds IL-18, and mice deficient in IL-18 have reduced cellular infiltration into the lungs and decreased levels of IFN-γ in the alveolar space early after influenza A virus infection (Liu et al. 2004). In a separate study, IL-18 knockout mice had a normal number of epitope-specific CD8 T cells but these were less capable of secreting IFN-γ, tumor necrosis factor (TNF)-α, and IL-2 (Denton et al. 2007). Collectively, these previous reports suggest that signaling through IL-1R and IL-18R has an important role in triggering the innate and adaptive immunity to generate a protective anti-influenza response. Our observations of increased expression of IL-18R, IL-1R, and TLRs in influenza A(H1N1)pdm09 patients with complex seizures suggest intriguing mechanisms by which the innate immune system contributes to the pathogenesis of febrile complex seizures due to influenza A(H1N1)pdm09.
KEGG pathway analysis also showed that the host gene expression in patients with complex seizures due to influenza A(H1N1)pdm09 could be involved in the MAPK-mediated activation of the TCR signaling pathway. MAPK family members, such as Jun N-terminal kinase, p38 MAPK, and ERK1/2, have important functions in antiviral immunity and are crucial for the expression of several proinflammatory cytokines, as well as the regulation of apoptosis (Kujime et al. 2000; Maruoka et al. 2003). Influenza virus infection strongly upregulates the phosphorylation of MAPK family members; thus, MAPK activation induces activator protein-1-dependent gene expression (Ludwig et al. 2001). In particular, influenza virus-mediated MAPK activation also contributes to cytokine production, such as TNF-α, IL-1β, and IL-8. Upregulation of the MAPK pathway of TCR signaling in complex seizures due to influenza virus might therefore lead to the overabundant production of proinflammatory cytokines and chemokines, which is known as the cytokine storm in the influenza-associated encephalopathy.
In contrast, GO analysis revealed there was no significant expressed transcript related to immune response in the blood from the complex seizures due to rotavirus gastroenteritis. Rotavirus infections induce virus-specific T cell and cytokine responses in children (Jaimes et al. 2002; Mäkelä et al. 2004; Rojas et al. 2003); however, there are few reports about the correlation between the cytokine responses and the occurrence of seizures due to rotavirus gastroenteritis. A previous study reported that rotavirus RNA was detected in the heart and liver and, in patients with seizures, in cerebrospinal fluid, suggesting a possible mechanism for extraintestinal complications (Lynch et al. 2003; Pang et al. 1996). Although rotavirus antigen or RNA is frequently detected in the cerebrospinal fluid or serum of rotavirus gastroenteritis patients with neurological manifestations, the antigenemia is also known to be commonly detected in rotavirus-infected children without any complications (Fischer et al. 2005; Sugata et al. 2008). None of the patients showed antigenemia in our study. Recently, the mutation of genes such as PRRT2 or SCN1A was reported to be one of the causes of benign infantile convulsions with mild gastroenteritis, which is a common condition characterized by repetitive brief seizures associated with symptoms of gastroenteritis in infants and young children. These studies suggest that complex seizures due to rotavirus gastroenteritis do not result from exaggerated immune responses different from those due to influenza A(H1N1)pdm09.
GO analysis also revealed that the most significant enriched GO term expressed in the complex seizures due to rotavirus was the cellular response to stress, but not those due to influenza A(H1N1)pdm09. Previous studies revealed that genes involved in stress response were induced by rotavirus in vitro (Bagchi et al. 2012; Gac et al. 2010); however, there has been no study about the gene expression related to the cellular response to stress in the blood of patients with rotavirus gastroenteritis. qRT-PCR validated that SOD2, OXR1, and HIGD1A were differentially expressed in the complex seizures due to rotavirus. The common characteristics of these genes are that their proteins are localized to the mitochondria, and that these genes are induced by oxidative stress or hypoxia. Mitochondria have important functions that include cellular ATP production, control of apoptotic/necrotic cell death, reactive oxygen species (ROS) formation and calcium homeostasis, and the major source of ROS within the cell is the mitochondria (Liu et al. 2002). These genes can function to protect cells against oxidative DNA damage produced by oxidative agents (superoxide anions and ROS) and prolong cell survival. Depletion of these genes results in mitochondrial dysfunction, leading to an increase in sensitivity to oxidative stress or hypoxia, and enhanced apoptosis. Dysregulation of expression of these genes in the blood could change the susceptibility to apoptosis and survival of blood cells. In our study, we were not able to clarify whether the response to oxidative stress was induced as the cause or the consequence of seizures due to rotavirus. However, we speculate that mitochondrial oxidative stress in the blood could be associated with the difference in pathogenesis in the seizures caused by rotavirus or influenza A(H1N1)pdm09.
In conclusion, our studies highlight the signatures of dysregulation of gene expression in complex seizure patients with influenza A(H1N1)pdm09 or rotavirus gastroenteritis and provide a framework for subsequent investigations into the different disease mechanisms. This study was limited by its small sample size for both the complex seizure and control patients, so the results must be interpreted with caution. Future studies will require careful case ascertainment and matching with healthy controls, as well as disease controls, to minimize the impact of confounding variables, such as age, sex, and pharmacological interventions, with a sufficiently large cohort of patients.
References
Abdul-Careem MF, Firoz Mian M, Gillgrass AE, Chenoweth MJ, Barra NG, Chan T, Al-Garawi AA, Chew MV, Yue G, van Roojen N, Xing Z, Ashkar AA (2011) FimH, a TLR4 ligand, induces innate antiviral responses in the lung leading to protection against lethal influenza infection in mice. Antiviral Res 92:346–355
Bagchi P, Nandi S, Chattopadhyay S, Bhowmick R, Halder UC, Nayak MK, Kobayashi N, Chawla-Sarkar M (2012) Identification of common human host genes involved in pathogenesis of different rotavirus strains: an attempt to recognize probable antiviral targets. Virus Res 169:144–153
Bekeredjian-Ding I, Roth SI, Gilles S, Giese T, Ablasser A, Hornung V, Endres S, Hartmann G (2006) T cell-independent, TLR-induced IL-12p70 production in primary human monocytes. J Immunol 176:7438–7446
Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, Forrest P, Gattas D, Granger E, Herkes R, Jackson A, McGuinness S, Nair P, Pellegrino V, Pettilä V, Plunkett B, Pye R, Torzillo P, Webb S, Wilson M, Ziegenfuss M (2009) Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA 302:1888–1895
Denton AE, Doherty PC, Turner SJ, La Gruta NL (2007) IL-18, but not IL-12, is required for optimal cytokine production by influenza virus-specific CD8+ T cells. Eur J Immunol 37:368–375
DiFazio MP, Braun L, Freedman S, Hickey P (2007) Rotavirus-induced seizures in childhood. J Child Neurol 22:1367–1370
Fischer TK, Ashley D, Kerin T, Reynolds-Hedmann E, Gentsch J, Widdowson MA, Westerman L, Puhr N, Turcios RM, Glass RI (2005) Rotavirus antigenemia in patients with acute gastroenteritis. J Infect Dis 192:913–919
Frobert E, Sarret C, Billaud G, Gillet Y, Escuret V, Floret D, Casalegno JS, Bouscambert M, Morfin F, Javouhey E, Lina B (2011) Pediatric neurological complications associated with the A(H1N1)pdm09 influenza infection. J Clin Virol 52:307–313
Gac M, Bigda J, Vahlenkamp TW (2010) Increased mitochondrial superoxide dismutase expression and lowered production of reactive oxygen species during rotavirus infection. Virology 404:293–303
Glaser CA, Winter K, DuBray K, Harriman K, Uyeki TM, Sejvar J, Gilliam S, Louie JK (2012) A population-based study of neurologic manifestations of severe influenza A(H1N1)pdm09 in California. Clin Infect Dis 55:514–520
Hart OM, Athie-Morales V, O’Connor GM, Gardiner CM (2005) TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol 175:1636–1642
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S (2004) Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529
Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, Saito Y, Takanashi J, Hirose S, Yamagata T, Yamanouchi H, Mizuguchi M (2012) Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev 34:337–343
Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol 162:3749–3752
Ichiyama T, Isumi H, Ozawa H, Matsubara T, Morishima T, Furukawa S (2003) Cerebrospinal fluid and serum levels of cytokines and soluble tumor necrosis factor receptor in influenza virus-associated encephalopathy. Scand J Infect Dis 35:59–61
Ito Y, Torii Y, Ohta R, Imai M, Hara S, Kawano Y, Matsubayashi T, Inui A, Yoshikawa T, Nishimura N, Ozaki T, Morishima T, Kimura H (2011) Increased levels of cytokines and high-mobility group box 1 are associated with the development of severe pneumonia, but not acute encephalopathy, in 2009 H1N1 influenza-infected children. Cytokine 56:180–187
Jaimes MC, Rojas OL, González AM, Cajiao I, Charpilienne A, Pothier P, Kohli E, Greenberg HB, Franco MA, Angel J (2002) Frequencies of virus-specific CD4(+) and CD8(+) T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J Virol 76:4741–4749
Kawada J, Kimura H, Kamachi Y, Nishikawa K, Taniguchi M, Nagaoka K, Kurahashi H, Kojima S, Morishima T (2006) Analysis of gene-expression profiles by oligonucleotide microarray in children with influenza. J Gen Virol 87:1677–1683
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Kawashima H, Morichi S, Okumara A, Nakagawa S, Morishima T, collaborating study group on influenza-associated encephalopathy in Japan (2012) National survey of pandemic influenza A (H1N1) 2009-associated encephalopathy in Japanese children. J Med Virol 84:1151–1156
Kedia S, Stroud B, Parsons J, Schreiner T, Curtis DJ, Bagdure D, Brooks-Kayal AR, Glode MP, Dominguez SR (2011) Pediatric neurological complications of 2009 pandemic influenza A (H1N1). Arch Neurol 68:455–462
Kolski H, Ford-Jones EL, Richardson S, Petric M, Nelson S, Jamieson F, Blaser S, Gold R, Otsubo H, Heurter H, MacGregor D (1998) Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994–1995. Clin Infect Dis 26:398–409
Kujime K, Hashimoto S, Gon Y, Shimizu K, Horie T (2000) p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J Immunol 164:3222–3228
Liu B, Mori I, Hossain MJ, Dong L, Takeda K, Kimura Y (2004) Interleukin-18 improves the early defence system against influenza virus infection by augmenting natural killer cell-mediated cytotoxicity. J Gen Virol 85:423–428
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787
Lloyd MB, Lloyd JC, Gesteland PH, Bale JF Jr (2010) Rotavirus gastroenteritis and seizures in young children. Pediatr Neurol 42:404–408
Ludwig S, Ehrhardt C, Neumeier ER, Kracht M, Rapp UR, Pleschka S (2001) Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J Biol Chem 276:10990–10998
Lynch M, Lee B, Azimi P, Gentsch J, Glaser C, Gilliam S, Chang HG, Ward R, Glass RI (2001) Rotavirus and central nervous system symptoms: cause or contaminant? Case reports and review. Clin Infect Dis 33:932–938
Lynch M, Shieh WJ, Tatti K, Gentsch JR, Ferebee-Harris T, Jiang B, Guarner J, Bresee JS, Greenwald M, Cullen S, Davies HD, Trevenen C, Zaki SR, Glass RI (2003) The pathology of rotavirus-associated deaths, using new molecular diagnostics. Clin Infect Dis 37:1327–1333
Mäkelä M, Marttila J, Simell O, Ilonen J (2004) Rotavirus-specific T-cell responses in young prospectively followed-up children. Clin Exp Immunol 137:173–178
Maruoka S, Hashimoto S, Gon Y, Nishitoh H, Takeshita I, Asai Y, Mizumura K, Shimizu K, Ichijo H, Horie T (2003) ASK1 regulates influenza virus infection-induced apoptotic cell death. Biochem Biophys Res Commun 307:870–876
Millichap JG, Millichap JJ (2006) Role of viral infections in the etiology of febrile seizures. Pediatr Neurol 35:165–172
Morishima T, Togashi T, Yokota S, Okuno Y, Miyazaki C, Tashiro M, Okabe N, Collaborative Study Group on Influenza-Associated Encephalopathy in Japan (2002) Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis 35:512–517
Newland JG, Laurich VM, Rosenquist AW, Heydon K, Licht DJ, Keren R, Zaoutis TE, Watson B, Hodinka RL, Coffin SE (2007) Neurologic complications in children hospitalized with influenza: characteristics, incidence, and risk factors. J Pediatr 150:306–310
Pang XL, Joensuu J, Vesikari T (1996) Detection of rotavirus RNA in cerebrospinal fluid in a case of rotavirus gastroenteritis with febrile seizures. Pediatr Infect Dis J 15:543–545
Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W, Fu T, Wang DY, Li Y, Wang HY, Wang RF (2005) Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science 309:1380–1384
Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J, Wightman P, Sato S, Akira S, Puel A, Casanova JL, Su B, Li X (2006) TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. J Biol Chem 281:21013–21021
Rojas OL, González AM, González R, Pérez-Schael I, Greenberg HB, Franco MA, Angel J (2003) Human rotavirus specific T cells: quantification by ELISPOT and expression of homing receptors on CD4+ T cells. Virology 314:671–679
Schmitz N, Kurrer M, Bachmann MF, Kopf M (2005) Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol 79:6441–6448
Shirey KA, Lai W, Scott AJ, Lipsky M, Mistry P, Pletneva LM, Karp CL, McAlees J, Gioannini TL, Weiss J, Chen WH, Ernst RK, Rossignol DP, Gusovsky F, Blanco JC, Vogel SN (2013) The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 497:498–502
Sugata K, Taniguchi K, Yui A, Miyake F, Suga S, Asano Y, Ohashi M, Suzuki K, Nishimura N, Ozaki T, Yoshikawa T (2008) Analysis of rotavirus antigenemia and extraintestinal manifestations in children with rotavirus gastroenteritis. Pediatrics 122:392–397
Surana P, Tang S, McDougall M, Tong CY, Menson E, Lim M (2011) Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur J Pediatr 170:1007–1015
Wang Y, Dennehy PH, Keyserling HL, Tang K, Gentsch JR, Glass RI, Jiang B (2007) Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J Virol 81:3904–3912
Xu J, Yang Y, Sun J, Ding Y, Su L, Shao C, Jiang B (2006) Expression of Toll-like receptors and their association with cytokine responses in peripheral blood mononuclear cells of children with acute rotavirus diarrhoea. Clin Exp Immunol 144:376–381
Yamashita N, Tsukahara H, Tsuge M, Nagaoka Y, Yashiro M, Saito Y, Fujii Y, Oka T, Morishima T (2013) Pathogenic mechanisms of influenza A(H1N1)pdm09 infection elucidated on gene expression profiling. Pediatr Int 55(5):572–577
Acknowledgments
This work was supported by research grants from the Japanese Ministry of Health, Labour and Welfare (20249053), and from the Japanese Ministry of Education, Culture, Sports, Science and Technology (22591179).
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuge, M., Oka, T., Yamashita, N. et al. Gene expression analysis in children with complex seizures due to influenza A(H1N1)pdm09 or rotavirus gastroenteritis. J. Neurovirol. 20, 73–84 (2014). https://doi.org/10.1007/s13365-013-0231-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-013-0231-5