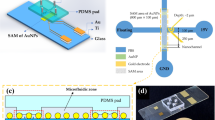
Abstract
Sample preconcentration is an important step that increases the accuracy of subsequent detection, especially for samples with extremely low concentrations. Due to the overlap of electrical double layers in a nanofluidic channel, the concentration polarization effect can be generated by applying an electric field. A nonlinear electrokinetic flow is induced, which results in the fast accumulation of proteins in front of the induced ionic depletion zone, the so-called exclusion- enrichment effect. In this way, a protein sample can be driven by electroosmotic flow and accumulated at a specific location. In the present study, a nanofluidic preconcentrator fabricated with the help of junction gap electric breakdown was integrated with microelectrodes for immunoassay. The preconcentration chip for proteins was fabricated using simple standard soft lithography with a polydimethylsiloxane replica. Human galectin-1 proteins from the cell lysate of T24 cells were concentrated and immunoassayed in the proposed microchip. The capability of the proposed microchip for concentrating multiple proteins from cell lysates and immunoassays after preconcentration was demonstrated. Immunosensing was evaluated by measurements of both fluorescence intensities and impedance, which proved the enhancement of preconcentration for immunoassay.
Similar content being viewed by others
References
Pu, Q., Yun, J., Temkin, H. & Liu, S. Ion-enrichment and ion-depletion effect of nanochannel structures. Nano Lett. 4, 1099–1103 (2004).
Duan, C., Wang, W. & Xie, Q. Review article: Fabrication of nanofluidic devices. Biomicrofluidics 7, 026501 (2013).
Wang, Y.C., Stevens, A.L. & Han, J. Million-fold preconcentration of proteins and peptides by nanofluidic filter. Anal. Chem. 77, 4293–4299 (2005).
Mao, P. & Han, J. Fabrication and characterization of 20 nm planar nanofluidic channels by glass-glass and glass-silicon bonding. Lab Chip 5, 837–844 (2005).
Lee, J.H., Song, Y.-A. & Han, J. Multiplexed proteomic sample preconcentration device using surface-patterned ion-selective membrane. Lab Chip 8, 596–601 (2008).
Sung, J.K. & Han, J. Self-sealed vertical polymeric nanoporous-junctions for high-throughput nanofluidic applications. Anal. Chem. 80, 3507–3511 (2008).
Wu, D. & Steckl, A.J. High speed nanofluidic protein accumulator. Lab Chip 9, 1890–1896 (2009).
Jen, C.-P., Amstislavskaya, T.G., Kuo, C.-C. & Chen, Y.-H. Protein preconcentration using nanofractures generated by nanoparticle-assisted electric breakdown at junction gaps. PLoS One 9, e102050 (2014).
Chiang, P.-J., Kuo, C.-C., Zamay, T.N., Zamay, A.S. & Jen, C.-P. Quantitative evaluation of the depletion efficiency of nanofractures generated by nanoparticle-assisted junction gap breakdown for protein concentration. Microelectron. Eng. 115, 39–45 (2014).
Jeong, H.L., Chung, S., Sung, J.K. & Han, J. Poly (dimethylsiloxane)-based protein preconcentration using a nanogap generated by junction gap breakdown. Anal. Chem. 79, 6868–6873 (2007).
Kim, S.M., Burns, M.A. & Hasselbrink, E.F. Electrokinetic protein preconcentration using a simple glass/ poly (dimethylsiloxane) microfluidic chip. Anal. Chem. 78, 4779–4785 (2006).
Liu, V., Song, Y.-A. & Han, J. Capillary-valve-based fabrication of ion-selective membrane junction for electrokinetic sample preconcentration in PDMS chip. Lab Chip 10, 1485–1490 (2010).
Lee, J.H. & Han, J. Concentration-enhanced rapid detection of human chorionic gonadotropin (hCG) on a Au surface using a nanofluidic preconcentrator. Microfluid. Nanofluidics 9, 973–979 (2010).
Ko, S.H. et al. Nanofluidic preconcentration device in a straight microchannel using ion concentration polarization. Lab Chip 12, 4472 (2012).
Chung, M., Kim, D. & Herr, A.E. Microchamber western blotting using poly-l-lysine conjugated polyacrylamide gel for blotting of sodium dodecyl sulfate coated proteins. Anal. Chem. 85, 7753–7761 (2013).
Banh, A. et al. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 71, 4423–4431 (2011).
Liu, F.-T. & Rabinovich, G.A. Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41 (2005).
Rabinovich, G.A. Galectin-1 as a potential cancer target. Br. J. Cancer 92, 1188–1192 (2005).
Van den Brûle, F., Califice, S. & Castronovo, V. Expression of galectins in cancer: a critical review. Glycoconj. J. 19, 537–542 (2004).
Scott, K. & Weinberg, C. Galectin-1: a bifunctional regulator of cellular proliferation. Glycoconj. J. 19, 467–477 (2004).
He, J. & Baum, L.G. Galectin interactions with extracellular matrix and effects on cellular function. Methods Enzymol. 417, 247–256 (2006).
Hsu, D.K. & Liu, F.-T. Regulation of cellular homeostasis by galectins. Glycoconj. J. 19, 507–515 (2004).
Thijssen, V.L.J.L. et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl. Acad. Sci. U.S.A. 103, 15975–15980 (2006).
Yang, R.Y. & Liu, F.T. Galectins in cell growth and apoptosis. Cell. Mol. Life Sci. 60, 267–276 (2003).
Barrow, H., Rhodes, J.M. & Yu, L.-G. The role of galectins in colorectal cancer progression. Int. J. Cancer 129, 1–8 (2011).
Camby, I. et al. Galectin-1 modulates human glioblastoma cell migration into the brain through modifications to the actin cytoskeleton and levels of expression of small GTPases. J. Neuropathol. Exp. Neurol. 61, 585–596 (2002).
Paz, A., Haklai, R., Elad-Sfadia, G., Ballan, E. & Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 20, 7486–7493 (2001).
Thrasher, J.B. & Crawford, E.D. Current management of invasive and metastatic transitional cell carcinoma of the bladder. J. Urol. 149, 957–972 (1993).
Stein, J.P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Cortessis, V.K., Siegmund, K., Xue, S., Ross, R.K. & Yu, M.C. A case-control study of cyclin D1 CCND1 870A—>G polymorphism and bladder cancer. Carcinogenesis 24, 1645–1650 (2003).
Peng, C.-C. et al. Antrodia camphorata extract induces replicative senescence in superficial TCC, and inhibits the absolute migration capability in invasive bladder carcinoma cells. J. Ethnopharmacol. 109, 93–103 (2007).
Cindolo, L. et al. galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int. J. Cancer 84, 39–43 (1999).
Danguy, A., Camby, I. & Kiss, R. Galectins and cancer. Biochim. Biophys. Acta 1572, 285–293 (2002).
Memon, A.A., Chang, J.W., Oh, B.R. & Yoo, Y.J. Identification of differentially expressed proteins during human urinary bladder cancer progression. Cancer Detect. Prev. 29, 249–255 (2005).
Memon, A.A. et al. Down-Regulation of S100C Is Associated with Bladder Cancer Progression and Poor Survival. Clin. Cancer Res. 11, 606–611 (2005).
Lee, J.H., Chung, S., Kim, S.J. & Han, J. Poly (dimethylsiloxane)-based protein preconcentration using a nanogap generated by junction gap breakdown. Anal. Chem. 79, 6868–6873 (2007).
Dill, K.A. & Shortle, D. Denatured States of Proteins. Annu. Rev. Biochem. 60, 795–825 (1991).
Jen, C.-P., Amstislavskaya, T.G., Chen, K.-F. & Chen, Y.-H. Sample preconcentration utilizing nanofractures generated by junction gap breakdown assisted by self-assembled monolayer of gold nanoparticles. PLoS One 10, e0126641 (2015).
Chuang, C.H. et al. Miniaturization of immunoassay by using a novel module-level immunosensor with polyaniline-modified nanoprobes that incorporate impedance sensing and paper-based sampling. Microfluid. Nanofluidics 16, 869–877 (2014).
Chuang, C.-H. et al. Lab on a chip for multiplexed immunoassays to detect bladder cancer using multifunctional dielectrophoretic manipulations. Lab Chip 15, 3056–3064 (2015).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, H.F., Amstislavskaya, T.G., Chen, PH. et al. Preconcentration-enhanced immunosensing for whole human cancer cell lysate based on a nanofluidic preconcentrator. BioChip J 10, 159–166 (2016). https://doi.org/10.1007/s13206-016-0203-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-016-0203-y