Abstract
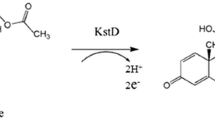
3-Ketosteroid-∆1-dehydrogenase (KstD), a key enzyme in microbial steroid catabolism, catalyzes the trans-axial elimination of the C1 and C2 hydrogen atoms of the A-ring from the polycyclic ring structure of 3-ketosteroids, and it was usually used to transform androst-4-ene-3,17-dione (AD) to produce androsta-1,4-diene-3,17-dione. Here, the KstD from Gordonia neofelifaecis was expressed efficiently in Escherichia coli. E. coli cells expressing KstD3gor were subjected to the investigation of dehydrogenation activity for different steroids. The results showed that KstD3gor has a clear preference for steroid substrates with 3-keto-4-ene configuration, and it exhibits higher activity towards steroid substrates carrying a small or no aliphatic side chain than towards substrates having a bulky side chain at the C-17 atom. The recombinant strain could efficiently convert androst-4,9(11)-dien-3,17-dione into androst-1,4,9(11)-trien-3,17-dione (with conversion rate of 96%). 1(2)-Dehydrogenation of androst-4,9(11)-dien-3,17-dione is one of the key steps in glucocorticoid production. To the best of our knowledge, this is the first study reporting on the conversion of androst-4,9(11)-dien-3,17-dione catalyzed by recombinant KstD; the expression system of KstD3gor reported here would have an impact in the industrial production of glucocorticoid in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Significant progress has been made over the last ten years in the use of enzymes and microorganisms for the manufacturing of complex chemical compounds and replacing multi-steps chemical syntheses. Actinobacteria are known as efficient biocatalysts of steroid bioconversion since 1913 (Tak 1942). However, the recent advances in genome sequencing and bioinformatics technologies provided tools for identification of new players in cholesterol bioconversion. Although steroids are highly resistant to biodegradation, many bacteria use them as a source of carbon and energy (García et al. 2012). Microbial transformation could be carried out under mild reaction conditions with excellent yields of products and remarkable regio- and stereo-selectivity, which is hardly available for chemical synthesis. Therefore, for producing novel steroidal drugs and generating active pharmaceutical ingredients, microbial transformation is employed as a novel, efficient and economical tool (Donova 2007; García et al. 2012; Yang et al. 2015). For example, side chains of phytosterol, a byproduct from soybeans, sugar and paper industries, can be selectively degraded by a process similar to the β-oxidation of fatty acids, yielding 17-ketosteroids (Wei et al. 2010). One of the products of this degradation, 9α-hydroxy-androst-4-ene-3,17-dione, and its ∆9-analog are considered as the most important intermediates for the synthesis of corticoids such as prednisolone, betamethasone, dexamethasone, and triamcinolone (Fokina and Donova 2003; Yuan et al. 2015). The efficiency of enzymatic processes and purity of their products have obvious advantages in comparison with multi-steps chemical syntheses of hormonal drugs. However, the development of steroid biotechnology requires further studies of microorganisms able to degrade/modify steroids as well as enzymes catalyzing these reactions on the molecular level (Yang et al. 2015).
The degradation of cholesterol or its derivatives begins with the transformation of cholesterol to cholest-4-en-3-one by a cholesterol oxidase (Shao et al. 2015). The subsequent catabolism involves elimination of the alkyl side chain followed by the opening of the rings A/B and rings C/D. A 3-ketosteroid Δ1-dehydrogenase (KstD) [EC 1.3.99.4], catalyzing the elimination of the hydrogen atoms of the C-1 and C-2 in the A-ring from the polycyclic ring structure of 3-ketosteroids, is a key enzyme in microbial steroid catabolism needed for the opening of the steroid B-ring (Fernández de Las Heras et al. 2012; Zhang et al. 2013). KstD is a FAD-dependent enzyme, the natural electron acceptor appears to be vitamin K2 (Choi et al. 1995a), and they can transfer electrons to N-methyl phenazolium sulfate. KstDs were found in various bacteria, including Mycobacterium sp., Rhodococus sp., Pseudomonas sp. and Arthrobacter sp. (Choi et al. 1995b; Molnar et al. 1995; van der Geize et al. 2000; Brzostek et al. 2005; Knol et al. 2008). They display a broad substrate spectrum. The KstD1SQ1 (KstD1 from Rhodococcus erythropolis SQ1) and KstD2SQ1 enzymes (KstD2 from Rhodococcus erythropolis SQ1) were specific for steroids with the 3-keto-4-ene structure such as 9α-hydroxy-androst-4-ene-3,17-dione (Knol et al. 2008). KstD3SQ1 (KstD3 from Rhodococcus erythropolis SQ1) had a clear preference for 3-ketosteroids with a saturated A-ring. The role of three KstDs from Rhodococcus ruber strain Chol-4 was studied in the steroid metabolism (Fernández de Las Heras et al. 2012).
KstD proteins were expressed in Escherichia coli (Wei et al. 2014), Bacillus subtilis (Zhang et al. 2013), Streptomyces lividans (Choi et al. 1995a), Rhodococcus erythropolis (Knol et al. 2008), Mycobacterium neoaurum (Morii et al. 1998), etc., and used to convert androst-4-ene-3,17-dione (AD) into androsta-1,4-diene-3,17-dione (Choi et al. 1995b; Morii et al. 1998; Knol et al. 2008; Zhang et al. 2013; Wei et al. 2014). An interesting possibility to use KstD for the 1(2)-dehydrogenation of androst-4,9(11)-dien-3,17-dione [4,9(11)-AD] (a step of the pathway for glucocorticoid production, as shown in Fig. 1) has not been tested.
Gordonia neofelifaecis NRRL B-59395 was initially isolated from fresh faeces of a clouded leopard (Neofelis nebulosa) for its ability to degrade cholesterol (Liu et al. 2011). In spite of a significant number of known actinobacteria that could degrade/modify steroids, only a few genomes of them have been completely sequenced to identify all sterol catabolic genes. We sequenced G. neofelifaecis NRRL B-59395 genome and found 5 putative genes encoding KstD (Ge et al. 2011; Li et al. 2014). We also studied the substrate specificity of these KstDs in our former study (Zhang et al. 2015). One enzyme, KstD3gor, had the broadest spectrum of substrate specificity, exhibiting activity to progesterone, 16α, 17α-epoxyprogesterone and cholest-4-en-3-one.
In this work, we cloned KstD3gor gene into E. coli vector, expressed the recombinant enzyme and characterized its enzyme specificity and selectivity. Our results indicated that KstD3gor could have a possible application for the production of androst-1,4,9(11)-trien-3,17-dione in the pharmaceutical industry.
Materials and methods
Chemicals
Androst-4,9(11)-dien-3,17-dione with purity of 99% was obtained from Zhejiang Shengzhou pharmaceutical Co. Ltd (China). 16α,17α-epoxyprogesterone, dehydroandrosterone, were obtained from XianJu Pharmaceutical Company Ltd. (Zhejiang Province, China) with purity of 98%. Cholesterol (purity ≥99%), progesterone (purity ≥99%), 5α-cholesteran-3β-ol (with purity of 92%), 3β-hydroxypregn-5-en-20-one (with purity of 92%), phenazine methosulfate (PMS, purity ≥90%), nitro blue tetrazolium (with purity of 98%), and dimethylformamide were purchased from Sigma (USA). Cholest-4-en-3-one, androst-4-en-3,17-dione (AD), (25R)-cholesten-26-oic acid with purity of 99% were synthesized and characterized using 1HNMR, 13C NMR, IR, and TOF–MS as it was done in our previous studies (Liu et al. 2011; Wu et al. 2015). (25R)-cholesten-26-oic acid was prepared as recommended (Martin et al. 2009). Restriction enzymes, dNTPs, Taq polymerase were purchased from TaKaRa Co. (Dalian, China).
Bacterial strains
Gordonia neofelifaecis (NRRL B-59395) was preserved in our laboratory (Ge et al. 2011) and cultivated in liquid LB medium (Luria–Bertani broth) at 37 °C. The strain had been originally isolated from the faeces of Neofelis nebulosa. Escherichia coli DH5α and E. coli BL21 (purchased from Transgen Biotech Co., Ltd, Beijing, China) were grown in LB broth or Super Optimal Broth (SOB, 2% peptone, 0.5% Yeast extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4) (Hanahan 1983) at 37 °C/200 rpm. Kanamycin (25 μg/mL) was added to the growth medium when necessary. For growing on solid medium, 2% (w/v) agar was added.
Construction and expression of KstD3gor in E. coli
The KstD3gor gene was amplified from previously cloned KstD3gor construct (Zhang et al. 2015) and cloned into the Nde I/BamH I restriction sites of pET-28a(+) vector (Novagen), giving the construct pET28a-KstD3. Recombinant DNA techniques were done according to standard protocols (Sambrook and Russell 2001). E. coli transformation was performed as described previously (Chung et al. 1989). The DNA was isolated using the Plasmid Mini Kit (Omega, USA) according to the manufacturer’s instructions. DNA sequence was verified by sequencing service provided by the Beijing Genome Institute Sequence Facility (Shenzhen, China).
Expression of KstD3gor in E. coli and preparation of clarified lysate
Recombinant KstD3gor protein was expressed in BL21(DE3) cells. E. coli cells transformed with pET28a-KstD3 construct were grown overnight (18 h) at 37 °C in 10 mL of LB medium containing 25 μg/mL kanamycin (the same concentration was used in all cultivation procedures) in a 50-mL Erlenmeyer flask. Ten mL of this culture was inoculated into 500 mL of LB medium containing antibiotic in a 2000-mL Erlenmeyer flask and incubated at 30 °C until the OD600 reached ~0.6. Then, the culture was induced with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG), and incubated with shaking (200 rpm) at 30 °C for 6 h. The cells were harvested by centrifugation at 10,000 rpm for 10 min. The cell pellet was washed for three times with 10 mL of chilled (4 °C) 50 mM Tris–HCl buffer (pH 7.0) (every time, centrifugation was performed at 10,000 rpm for 10 min at 4 °C), resuspended in 10 mL of the same buffer and sonicated using an ultrasonic homogenizer (JY92-II, Scientz Biotechnology Co. Ltd., China) in an ice bath, with 90 cycles of 5 s on and 5 s off at 220 W. The cell lysates were centrifuged for 30 min at 18,000 rpm and 4 °C. The supernatant (clarified lysate) and the washed cell pellet were used for KstD activity gel assay or biochemical assays for the measurements of bioconversion of steroid substrates.
KstD activity staining on native PAGE
The cell extract was used for analysis of KstD activity on native 12.5% PAGE (Knol et al. 2008). The KstD activity was visualized by incubating native gels in 100 mL 50 mM Tris–HCl buffer (pH 7.0) containing 3.1 mg phenazine methosulphate, 2.9 mg androst-4-en-3, 17-dione dissolved in ethanol and 41 mg nitroblue tetrazolium (NBT) dissolved in 70% dimethylformamide. The native gels were stained for several hours until the appearance of distinct purple-colored bands of the product of the reaction, formazan on the gel. Then, the staining was stopped by replacing the staining solution with 100 mL of 10% (v/v) acetic acid.
Preparation of steroid-methyl-β-cyclodextrin (Me-β-CD) complex (steroid-complex)
Steroid-methyl-β-cyclodextrin (Me-β-CD) complex (steroid-complex) was prepared by the co-evaporation method as previously reported (Manosroi et al. 2008). Briefly, 0.2 mol of methyl-β-cyclodextrin and 0.1 mol of steroid substrates were dispersed in 95% ethanol and stirred with a speed of 200 rpm for 4 h at 37 °C. Then, ethanol was evaporated at 60 °C on a Buchi model R-210 rotary evaporator (0.09 Mpa, with a speed of 60 rpm at 65 °C) (The same parameters of the instrument were used throughout the study to concentrate extraction samples).
Bioconversion of steroid substrates by KstD3gor-expressing E. coli cells and extracts
The bioconversion of steroid substrate was performed in 100 mL Erlenmeyer flasks with the clarified lysates or E. coli cells. To prepare E. coli cells, the cells expressing KstD3gor were harvested by centrifugation from 4 mL fresh culture, and resuspended in 20 mL Tris–HCl buffer (pH 7.0) to the concentration of 5 × 107 CFU/mL. The clarified lysates from 4 mL fresh culture were prepared as described above. Then, steroid complex was added to the 20 mL of cell suspension or clarified lysate. The final concentration of each substrate was 2 g/L. The reaction mixture was incubated at 37 °C with shaking at 200 rpm for 10–24 h. Steroids were extracted from the medium by adding the equivalent volume of ethyl acetate; after phase separation, the upper organic phase was analyzed by thin-layer chromatography (TLC). The TLC was performed on 0.25 mm-thick silica gel G (silica gel 254, Qingdao Haiyang Chemical Co., Ltd.) with cyclohexane/ethyl acetate (7:3, v/v) as the mobile phase. 10 μL sample of organic phase was applied on TLC plate. The products of the enzymatic reaction were visualized by spraying a mixture of sulfuric acid and methanol (1:6, v/v) on the plates and heating them at 100 °C until the colors developed. The extraction procedure was repeated three times, and the steroid extract was dried by reduced pressure distillation (0.09 MPa, 65 °C) and further separated by high-performance liquid chromatography (HPLC) (Shimadzu, Kyoto, Japan). To perform HPLC analysis, samples were diluted to an appropriate concentration (about 1 mg/mL) with methanol and filtered through 0.22 μm pore-size membranes. HPLC analysis was performed on an Alltima C18 column (250 × 4.6 mm, 5 μm, Alltech, USA) under the control of an HPLC system (Shimadzu, Japan) equipped with an LC-20AB HPLC pump system and SPD-20A UV detector. The HPLC was performed using methanol–water (6:4, v/v) as the mobile phase at a flow rate of 1 mL/min.
The conversion rate of sterol was calculated according to the following formula:
Purification and spectroscopic analysis of transformation products
Purification of the products of bioconversion was performed as previously described (Liu et al. 2011). Briefly, 500 mL of the reaction mixture was extracted with 500 mL ethyl acetate (v/v) at room temperature for 30 min. The organic phase was collected and evaporated under reduced pressure. The crude gum was dissolved in ethyl acetate, and applied to a silica gel column (2.5 cm × 30 cm), eluted with acetone/ethyl acetate (the eluent system consisted of gradient mixtures of chloroform and acetone and ethyl acetate), at a flow rate of 1 mL/min. Fractions of 10 mL were collected. The fractions containing the same steroid intermediates were pooled and concentrated in a rotary evaporator. Finally, the product was recrystallized from anhydrous alcohol and was subjected to 1HNMR, 13C NMR, or TOF–MS analysis.
Results
Expression of the catalytically active KstD3gor in E. coli cells
To expand our knowledge about KstD3gor from G. neofelifaecis, the gene of this enzyme was sub-cloned into a commercial E. coli vector pET28a(+) and recombinant KstD3gor protein was expressed in BL21(DE3) cells. The calculated molecular mass of KstD3gor (57 kDa) corresponded to the most abundant band on the 10% SDS-PAGE in the sample with induced expression of the protein (Fig. 2a, lane 3). A significant amount of KstD3gor was expressed as a soluble protein (Fig. 2a, lanes 3, 4). Decreasing the temperature (from 30 to 25 °C) and increasing the induction time (from 4 to 6 h) led to a high level of recombinant protein expression.
Expression of catalytically active KstD3gor in BL21(DE3) cells. a Expression of the protein analyzed by 10% SDS-PAGE: lane 1 molecular weight marker; lane 2 total cell lysate of BL21(DE3)/pET28a-KstD3 before IPTG induction; lane 3 The total cell lysate of the same culture as in lane 2 after IPTG induction; lane 4, the clarified lysate of the sample in lane 3. b KstD activity visualized on native PAGE gel loaded with total cell extracts: lane 1 clarified lysate of BL21(DE3)/pET28a cells (negative control); lane 2 clarified lysate of BL21(DE3) cells expressing KstD3gor after 4 h induction; lane 3 clarified lysate of the same cells as in lane 2 after 8 h induction
KstD activity staining on native PAGE confirmed the expression of catalytically active enzyme. The clarified lysates were prepared and assayed by native PAGE as described in Sect. “Materials and methods”. The electron transfer from AD via phenazine methosulphate to NBT catalyzed by KstD3gor resulted in the formation of a purple-colored product of the reaction, formazan (Fig. 2b, lanes 2, 3). Meanwhile, no activity was detected in the negative control sample (Fig. 2b, lane 1).
Bioconversion of steroid substrates by KstD3gor-expressing E. coli cells and clarified lysates
The E. coli cells expressing KstD3gor were tested in the dehydrogenation reaction of androst-4,9(11)-dien-3,17-dione. The structures of the products were determined using spectroscopic techniques including 1HNMR, 13C NMR and TOF–MS. The TOF–MS spectra are shown in Fig. 3a, TOF–MS m/z: 283.17[M+H]+, 305.15[M+Na]+. 1H NMR spectrum in CDCl3 (δ, ppm): 0.89 (3H, s, H-18), 1.36 (3H, s, H-19), 5.59 (br t, 1H, H-11), 6.09 (1H, s, H-4), 6.30 (1H, dd, J = 10.2, 1.9 Hz, H-2), 7.27 (1H, d, J = 10 Hz, H-1). 13C NMR spectrum in CDCl3 (δ, ppm): 154.3 (C-1), 127.2 (C-2), 186.0 (C-3), 123.8 (C-4), 166.1 (C-5), 33.7 (C-6), 31.6 (C-7), 34.2 (C-8), 143.4 (C-9), 45.9 (C-10), 119.8(C-11), 33.5 (C-12), 46.0 (C-13), 48.1 (C-14), 26.4 (C-15), 36.1 (C-16), 220.2 (C-17), 13.7 (C-18), 22.8 (C-19). The result showed that the product reduces two mass units from androst-4,9(11)-dien-3,17-dione (284.39), suggesting that dehydrogenation reaction has occurred at the C-1 and C-2 in the A-ring.
In a typical bioconversion experiment, 500 mL of KstD3gor-expressing E. coli cells supplemented with 2 g/L androst-4,9(11)-dien-3,17-dione produced 1.94 g/L androst-1,4,9(11)-trien-3,17-dione, the reaction mixture was incubated for 16 h, and the conversion ratio of androst-1,4,9(11)-trien-3,17-dione in the products reached the highest conversion rate (96%). The property of KstD3gor-expressing E. coli cells to catalyze the dehydrogenation of androst-1,4,9(11)-trien-3,17-dione was confirmed by HPLC using androst-4,9(11)-dien-3,17-dione as a substrate (as shown in Fig. 4). The similar results were obtained for the clarified lysates (data not shown).
HPLC analysis of steroid substrates bioconversion by KstD3gor-expressing E. coli cells. a The standard samples of androst-4,9(11)-dien-3,17-dione. b The standard samples of androst-1,4,9(11)-trien-3,17-dione. c The product by KstD3gor-expressing E. coli cells. d The product by BL21(DE3)/pET28a cells
Substrate preference and selectivity of KstD3gor
To characterize the substrate preferences of the recombinant KstD3gor, nine steroid substrates were used in the dehydrogenation reaction catalyzed by KstD3gor-expressing cells (Table 1). The structures of the products were determined using spectroscopic techniques, The TOF–MS spectra of the transformed products from progesterone are shown in Fig. 3b, TOF–MS m/z: 313.21 [M+H]+, 335.19[M+Na]+. 1H NMR spectrum in CDCl3 (δ, ppm): 0.70 (3H, s, H-18), 1.24 (3H, s, H-19), 2.13 (3H, s, H-21), 6.09 (1H, s, H-4), 6.30 (1H, dd, J = 10.2, 1.9 Hz, H-2), 7.04 (1H, d, J = 10 Hz, H-1). 13C NMR spectrum in CDCl3 (δ, ppm): 155.6 (C-1), 127.4 (C-2), 186.3 (C-3), 123.6 (C-4), 168.9 (C-5), 33.5 (C-6), 31.1 (C-7), 35.3 (C-8), 55.4 (C-9), 38.5 (C-10), 22.7 (C-11), 42.9 (C-12), 43.9 (C-13), 51.9 (C-14), 22.8 (C-15), 32.5 (C-16), 62.9 (C-17), 13.8 (C-18), 18.7 (C-19), 209.2 (C-20), 24.5 (C-21). The 1HNMR, 13C NMR, and TOF–MS spectroscopic data suggested that progesterone (pregn-4-ene-3,20-dione) was transformed to pregn-1,4-diene-3,20-dione. The transformed products of androst-4-ene-3,17-dione and 16α,17α-epoxyprogesterone were identified as androst-1,4-diene-3,17-dione and 16α,17-epoxypregn-1,4-diene-3,20-dione, respectively; the spectroscopic data were as the same as our previous reported work (Liu et al. 2011; Zhang et al. 2015).
As shown in Table 1, KstD3gor-expressing cells exhibited the dehydrogenase activity towards androst-4-en-3,17-dione, androst-4,9(11)-dien-3,17-dione, progesterone and 16α,17α-epoxyprogesterone (Fig. 5). Androst-4-en-3,17-dione, androst-4,9(11)-dien-3,17-dione appear to be the preferred KstD3gor substrate, with conversion rate of 96%, respectively. However, KstD3gor-expressing cells had no ability of catalyzing the conversion of cholest-4-en-3-one, (25R)-cholesten-26-oic acids, dehydroandrosterone, 5α-cholesteran-3β-ol, 3β-hydroxypregn-5-en-20-one. No KstD activity was observed in negative control (data not shown).
TLC analysis of several steroid substrates conversion by KstD3gor-expressing cells. Lane 1 androst-4-en-3, 17-dione; lane 2 the conversion products of androst-4-en-3, 17-dione; lane 3 androst-4,9(11)-dien-3,17-dione; lane 4 the conversion products of androst-4,9(11)-dien-3,17-dione; lane 5 cholest-4-en-3-one; lane 6 the conversion products of cholest-4-en-3-one; lane 7 progesterone; lane 8 the conversion products of progesterone; lane 9 16α,17α-epoxyprogesterone; lane 10 the conversion products of progesterone 16α,17α-epoxyprogesterone
Discussion
KstDs are widely found in actinobacteria as they play an important role in microbial steroid degradation. Different isoforms of the enzyme have been found exhibiting different substrate specificity and having different roles that could be strain-dependent (Knol et al. 2008). The genome of R. ruber strain Chol-4 contains three different KstD genes, and two of them contribute to cholesterol catabolism (Fernández de Las Heras et al. 2012). Rhodococcus jostii RHA1 KstDs display a quite different substrate specificity (Knol et al. 2008).
The sterol catabolic genes are highly conserved in G. neofelifaecis, R. jostii RHA1, and Mycobacterium tuberculosis, and mainly organized in three specific clusters. In our previous research, the substrate preference of five G. neofelifaecis KstDs was investigated preliminarily (Zhang et al. 2015). Here, the KstD3gor was biochemically characterized in detail.
A phylogenetic tree (Fig. 6) of the characterized KstDs revealed that they share high similarity with other bacterial KstD homolog. They clustered into at least four distinct groups (Knol et al. 2008). KstD3gor showed 63.6 and 44.7% amino acid identity with R. ruber KstD1 and R. erythropolis KstD1, correspondingly. Both KstD3gor and R. erythropolis KstD1 prefer 3-ketosteroids with a saturated A-ring as substrates.
Since KstD3gor activity was not detected with trans-dehydroandrosterone which has no aliphatic side chain, the 3-keto-4-ene configuration of the steroid substrate appears to be important for KstD3gor activity. KstD3gor efficiently catalyzed the Δ1-dehydrogenation of steroid substrates carrying a small or no aliphatic side chain, compared to the steroids with a bulky side chain at the C-17 atom (i.e., cholest-4-en-3-one, (25R)-cholesten-26-oic acid). The conversion rate of 16α,17α-epoxyprogesterone was about 3.5-fold lower compared to that of progesterone, showing that besides the length of the 17-alkyl side chain, the additional molecular group in D-ring affects the catalytic activity of KstD3gor. The 4,9(11)-dehydrogenation of C-ring had no effect on the KstD3gor activity.
Cholest-4-en-3-one is the second intermediate product of cholesterol catabolism (Knol et al. 2008), and (25R)-cholesten-26-oic acids are the first product of the side-chain degradation, but KstD3gor, KstD1-3SQ1 and KstDH37Rv could not catalyze their 1(2)-dehydrogenation. Thus, these KstDs could act after elimination of the sterol side-chain.
The introduction of the C1-C2 double bond into ring A of steroid could improve the biological activity of the original steroid substrate (i.e., prednisone and prednisolone) (Fokina and Donova 2003). The enzyme KstD catalyzing this reaction has been widely characterized during the past two decades. We studied the KstD3gor 1(2)-dehydrogenation activity for steroids with different A-ring, B-ring, C-ring and D-ring modifications. The results showed that KstD3gor has high activity towards both 4-AD and 4-pregnene-3,20-dione (progesterone) which contain 3-keto-4-ene configuration and a small or no aliphatic side chain at the C-17 atom. It appears to be different from the KstD1SQ1 (KstD1 from Rhodococcus erythropolis SQ1) which shows high activity towards 4-AD but low activity towards 4-pregnene-3,20-dione (Knol et al. 2008). While KstD3SQ1 had a clear preference for 3-ketosteroids with a saturated A-ring, the 1(2)-dehydrogenation of androst-4,9(11)-dien-3,17-dione is one step of the pathway for glucocorticoid production (Fig. 1). This pathway is considered to be an effective method to produce fluorocorticoids (such as dexamethasone, betamethasone, triamcinolone, etc.) and other valuable steroid drugs (Fokina and Donova 2003). But the application of KstD for the 1(2)-dehydrogenation of androst-4,9(11)-dien-3,17-dione [4,9(11)-AD] has not been tested. The conversion rate of androst-4,9(11)-dien-3,17-dione to androst-1,4,9(11)-trien-3,17-dione by the KstD3gor-expressing cells was more than 96%. Thus, this work provided a candidate, KstD3gor-expressing cells, for the industrial conversion of steroids to their 1(2)-dehydro-analogs.
In summary, the KstD3gor from G. neofelifaecis was expressed efficiently in a soluble form in E. coli. The KstD3gor-expressing cells effectively converted androst-4,9(11)-dien-3,17-dione and androst-4-en-3, 17-dione to androst-1,4,9(11)-trien-3,17-dione and androst-1,4-en-3,17-dione, respectively. Both of them can be recommended as substrate for 1(2)-dehydrogenation by KstD3gor-expressing E. coli in the commercial production of 9-halogenated steroids.
References
Brzostek A, Sliwiński T, Rumijowska-Galewicz A et al (2005) Identification and targeted disruption of the gene encoding the main 3-ketosteroid dehydrogenase in Mycobacterium smegmatis. Microbiol 151:2393–23402
Choi KP, Molnár I, Murooka Y (1995a) Secretory overproduction of Arthrobacter simplex 3-ketosteroid-△1-dehydrogenase by Streptomyces lividans with a multi-copy shuttle vector. Appl Microbiol Biotechnol 43:1044–1049
Choi KP, Molnár I, Yamashita M et al (1995b) Purification and characterization of the 3-ketosteroid-delta 1-dehydrogenase of Arthrobacter simplex produced in Streptomyces lividans. J Biochem 117:1043–1049
Chung CT, Niemela SL, Miller RH (1989) One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci 86:2172–2175
de Las Fernández, Heras L, van der Geize R, Drzyzga O et al (2012) Molecular characterization of three 3-ketosteroid-Δ(1)-dehydrogenase isoenzymes of Rhodococcus ruber strain Chol-4. J Steroid Biochem Mol Biol 132:271–281
Donova MV (2007) Transformation of steroids by actinobacteria: a review. Appl Biochem Microbiol 43:1–14
Fokina VV, Donova MV (2003) 21-Acetoxy-pregna-4(5),9(11),16(17)-triene-21-ol-3,20-dione conversion by Nocardioides simplex VKM Ac-2033D. J Steroid Biochem Mol Biol 87:319–325
García JL, Uhía I, Galán B (2012) Catabolism and biotechnological applications of cholesterol degrading bacteria. Microbiol Biotechnol 5:679–699
Ge F, Li W, Chen G et al (2011) Draft genome sequence of Gordonia neofelifaecis NRRL B-59395, a cholest-4-en-3-one-degrading actinomycete. J Bacteriol 193:5045–5046
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Knol J, Bodewits K, Hessels GI et al (2008) 3-Keto-5alpha-steroid Delta(1)-dehydrogenase from Rhodococcus erythropolis SQ1 and its orthologue in Mycobacterium tuberculosis H37Rv are highly specific enzymes that function in cholest-4-en-3-one catabolism. Biochem J 410:339–346
Li WJ, Ge F, Zhang Q et al (2014) Identification of gene expression profiles in the actinomycete Gordonia neofelifaecis grown with different steroids. Genome 57:345–353
Liu YC, Chen YG, Ge F et al (2011) Efficient biotransformation of cholest-4-en-3-one to androsta-1,4-diene-3, 17-dione by a newly isolated actinomycete Gordonia neofelifaecis. World J Microbiol Biotechnol 27:4759–4765
Manosroi A, Saowakhon S, Manosroi J (2008) Enhancement of androstadienedione production from progesterone by biotransformation using the hydroxypropyl-beta-cyclodextrin complexation technique. J Steroid Biochem Mol Biol 108:132–136
Martin R, Schmidt AW, Theumer G et al (2009) Synthesis and biological activity of the (25R)-cholesten-26-oic acids–ligands for the hormonal receptor DAF-12 in Caenorhabditis elegans. Org Biomol Chem 7:909–920
Molnar I, Choi KP, Yamashita M et al (1995) Molecularcloning, expression in Streptomyces livdans, and analysis of a gene cluster from Arthrobacter simplex encoding 3-ketosteroid-Δ1-dehydrogenase, 3-ketosteroid-Δ5-isomerase and a hypothetical regulatory protein. Mol Microbiol 15:895–905
Morii S, Fujii C, Miyoshi T et al (1998) 3-Ketosteroid-delta1-dehydrogenase of Rhodococcus rhodochrous: sequencing of the genomic DNA and hyperexpression, purification, and characterization of the recombinant enzyme. J Biochem 124:1026–1032
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Shao ML, Rao ZM, Zhang X et al (2015) Bioconversion of cholesterol to 4-cholesten-3-one by recombinant Bacillus subtilis expressing choM gene encoding cholesterol oxidase from Mycobacterium neoaurum JC-12. J Chem Technol Biotechnol 90:1811–1820
Tak JD (1942) On bacteria decomposing cholesterol. Antonie Van Leeuwenhoek 8:32–40
van der Geize R, Hessels GI, van Gerwen R et al (2000) Targeted disruption of the kstD gene encoding 3-ketosteroid D1-dehydrogenase isoenzyme of Rhodococcus erythropolis SQ1. Appl Environ Microbiol 66:2029–2036
Wei W, Wang FQ, Fan SY et al (2010) Inactivation and augmentation of the primary 3-ketosteroid-{delta}1-dehydrogenase in Mycobacterium neoaurum NwIB-01: biotransformation of soybean phytosterols to 4-androstene-3,17-dione or 1,4-androstadiene-3,17-dione. Appl Environ Microbiol 76:4578–4582
Wei W, Fan SY, Wang FQ et al (2014) Accumulation of androstadiene-dione by overexpression of heterologous 3-ketosteroid Δ1-dehydrogenase in Mycobacterium neoaurum NwIB-01. World J Microbiol Biotechnol 30:1947–1954
Wu K, Li W, Song J et al (2015) Production, purification, and identification of cholest-4-en-3-one Produced by cholesterol oxidase from Rhodococcus sp. in aqueous/organic biphasic system. Biochem Insights 16(Suppl 1):1–8
Yang M, Lu R, Guja KE et al (2015) Unraveling cholesterol catabolism in Mycobacterium tuberculosis: ChsE4-ChsE5 α2β2 Acyl-CoA dehydrogenase initiates β-oxidation of 3-oxo-cholest-42-en-26-oyl CoA. ACS Infect Dis 13:110–125
Yuan JD, Chen GY, Cheng JS et al (2015) Accumulation of 9α-hydroxy-4-androstene-3,17-dione by co-expressing kshA and kshB encoding component of 3-ketosteroid-9α-hydroxylase in Mycobacterium sp. NRRL B-3805. Chin J Biotechnol 31:523–533
Zhang W, Shao M, Rao Z et al (2013) Bioconversion of 4-androstene-3,17-dione to androst-1,4-diene-3,17-dione by recombinant Bacillus subtilis expressing ksdd gene encoding 3-ketosteroid-Δ1-dehydrogenase from Mycobacterium neoaurum JC-12. J Steroid Biochem Mol Biol 135:36–42
Zhang QY, Ren Y, He JH et al (2015) Multiplicity of 3-ketosteroid Δ1-dehydrogenase enzymes in Gordonia neofelifaecis NRRL B-59395 with preferences for different steroids. Ann Microbiol 65:1961–1971
Acknowledgements
This study was funded by the National Natural Science Foundation of China (No. 31571285), the Sichuan provincial Science & Technology Department (2008JY0103-1, 2009JY0067), and the Sichuan provincial Department of Education (13ZA0145), College students innovation and entrepreneurship training program (201310636026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
W. Wang and F. Ge contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, W., Ge, F., Ma, C. et al. Heterologous expression and characterization of a 3-ketosteroid-∆1-dehydrogenase from Gordonia neofelifaecis and its utilization in the bioconversion of androst-4,9(11)-dien-3,17-dione. 3 Biotech 7, 19 (2017). https://doi.org/10.1007/s13205-017-0601-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0601-4