Abstract
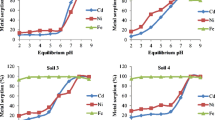
The chemical associations of Cd, Cu, Pb, and Zn in four mine soil samples from the Amizour-Bejaia Pb/Zn mine (Algeria) have been investigated by a five-step sequential extraction procedure. Although Cd preferentially binds to carbonates, Cu, Pb, and Zn are mainly associated with the organic and reducible fractions. Batch adsorption experiments with either mono- or multi-metallic solutions are described with the Freundlich isotherm model. Whatever the nature of the soil sample, the sorption behavior for each given metal except Pb is very similar, indicating that the binding sites at the soil surface are progressively occupied by the metal from the solution. On each soil sample, the decreasing order of sorption can be established as Pb >> Cu > Cd > Zn. When the four metals are simultaneously applied to each soil sample, their specific behavior is strongly affected by their interactions and/or competition for the available surface sites: we generally observed isotherm curves with a slight maximum before the plateau at higher solution concentration. Although Cu is only slightly affected by the other metals, in the case of Pb, Cd, and Zn, the sorbed amounts strongly decreased.



Similar content being viewed by others
References
Arenas-Lago D, Lago-Vila M, Rodriguez-Seijo A, Andrade ML, Vega FA (2014) Risk of metal mobility in soils from a Pb/Zn depleted mine (Lugo, Spain). Environ Earth Sci 72:2541–2556
Arias M, Pérez-Novo C, Osorio F, López E, Soto B (2005) Adsorption and desorption of copper and zinc in the surface layer of acid soils. J Colloid Interface Sci 288:21–29
Ash C, Tejnecký V, Šebek O, Houška J, Chala AT, Drahota P, Drábek O (2015) Redistribution of cadmium and lead fractions in contaminated soil samples due to experimental leaching. Geoderma 241-242:126–135
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18
Covelo EF, Andrade ML, Vega FA (2007a) Competitive sorption and desorption of heavy metals by individual soil components. J Hazard Mater 140:308–315
Covelo EF, Vega FA, Andrade ML (2007b) Simultaneous sorption and desorption of Cd, Cr, Cu, Ni, Pb, and Zn in acid soils: I. Selectivity sequences J Hazard Mater 147:852–861
Covelo EF, Vega FA, Andrade ML (2007c) Simultaneous sorption and desorption of Cd, Cr, Cu, Ni, Pb, and Zn in acid soils: II. Soil ranking and influence of soil characteristics. J Hazard Mater 147:862–870
Delmas C, Larpin L, Legret M, Astruc M (2002) Mobility and adsorption capacity of Pb and Zn in a polluted soil from a road environment: laboratory batch experiments. Environ Technol 23:381–390
Evans LJ (1989) Chemistry of metal retention by soils. Environ Sci Technol 23:1046–1056
Fernandez E, Jimenez R, Lallena AM, Aguilar J (2004) Evaluation of the BCR sequential extraction procedure applied for two unpolluted Spanish soils. Environ Pollut 131:355–364
Fields S (2003) The earth’s open wounds: abandoned and orphaned mines. Environ Health Perspect 111:A154–A161
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Green-Pedersen H, Jensen BT, Pind N (1997) Nickel adsorption on MnO2, Fe(OH)3, montmorillonite, humic acid and calcite: a comparative study. Environ Technol 18:807–815
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Iavazzo P, Adamo P, Boni M, Hillier S, Zampella M (2012) Mineralogy and chemical forms of lead and zinc in abandoned mine wastes and soils: an example from Morocco. J Geochem Explor 113:56–67
ISO (1995) Soil quality. Determination of organic and total carbon after dry combustion (elementary analysis). ISO, vol. 10694
Limousin G, Gaudet JP, Charlet L, Szenknect S, Barthes V, Krimissa M (2007) Sorption isotherms: a review on physical bases, modeling and measurement. Appl Geochem 22:249–275
Marcus Y (1985) Ion solvation. Wiley, Chichester
Mench M, Vangronsveld J, Didier V, Clijsters H (1994) Evaluation of metal mobility, plant availability and immobilization by chemical agents in a limed-silty soil. Environ Pollut 86:279–286
Merabet D, Mahtout L (1999) Essais d’enrichissement par flottation du minerai plombo-zincifère d’Amizour. Chron Rech Minière 67(535):59–63
Misono M, Ochiai E, Saito Y, Yoneda Y (1967) A new dual parameter scale for the strength of Lewis acids and bases with the evaluation of their softness. J Inorg Nucl Chem 29:2685–2691
Morera MT, Echeverria JC, Mazkiaran C, Garrido JJ (2001) Isotherm and sequential extraction procedures for evaluating sorption and distribution of heavy metals in soils. Environ Pollut 113:135–144
Mouni L, Merabet D, Robert D, Bouzaza A (2009) Batch studies for the investigation of the sorption of the heavy metals Pb2+ and Zn2+ onto Amizour soil (Algeria). Geoderma 154:30–35
Pansu M, Gautheyrou J (2003) L'analyse du sol : minéralogique, organique et minérale. Springer, Paris
Pascaud G, Leveque T, Soubrand M, Boussen S, Joussein E, Dumat C (2014) Environmental and health risk assessment of Pb, Zn, As and Sb in soccer field soils and sediments from mine tailings: solid speciation and bioaccessibility. Environ Sci Pollut Res 21:4254–4264
Plassard F, Winiarski T, Petit-Ramel M (2000) Retention and distribution of three heavy metals in a carbonated soil: comparison between batch and unsaturated column studies. J Contam Hydrol 42:99–111
Rodriguez L, Ruiz E, Alonso-Azcarate J, Rincon J (2009) Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. J Environ Manag 90:1106–1116
Sastre J, Rauret G, Vidal M (2007) Sorption–desorption tests to assess the risk derived from metal contamination in mineral and organic soils. Environ Int 33:246–256
Serrano S, Garrido F, Campbell CG, Garcia-Gonzalez MT (2005) Competitive sorption of cadmium and lead in acid soils of Central Spain. Geoderma 124:91–104
Stumm W, Morgan JJ (1996) Aquatic chemistry. Wiley, New York
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Trakal L, Komárek M, Száková J, Tlustoš P, Tejnecký V, Drábek O (2012) Sorption behavior of Cd, Cu, Pb, and Zn and their interactions in phytoremediated soil. Int J Phytoremed 14:806–819
USDA (1987) United States Department of Agriculture, Soil Conservation Service. Soil Mechanics Level I, Module 3: USDA Textural Soil Classification—Study Guide; freely available at: www.wcc.nrcs.usda.gov/ftpref/wntsc/H&H/training/soilsOther/soil-USDA-textural-class.pdf . A ‘Soil Texture Calculator’ is available as free Excel software at: http://www.nrcs.usda.gov/wps/portal/nrcs/detail//?cid=nrcs142p2_054167 (accessed 15 April, 2015)
Vega FA, Covelo EF, Andrade ML (2006) Competitive sorption and desorption of heavy metals in mine soils: influence of mine soil characteristics. J Colloid Interface Sci 298:582–592
Violante A, Cozzolino V, Perelomov L, Caporale AG, Pigna M (2010) Mobility and bioavailability of heavy metals and metalloids in soil environments. J Soil Sci Plant Nutr 10:268–292
Wang LJ, Lu XW, Li LY, Ren CH, Luo DC, Chen JG (2015) Content, speciation and pollution assessment of Cu, Pb and Zn in soil around the lead-zinc smelting plant of Baoji, NW China. Environ Earth Sci 73:5281–5288
Waterlot C, Bidar G, Pelfrêne A, Roussel H, Fourrier H, Douay F (2013) Contamination, fractionation and availability of metals in urban soils in the vicinity of former lead and zinc smelters, France. Pedosphere 23:143–159
Zemanová V, Trakal L, Ochecová P, Száková J, Pavlíková D (2014) A model experiment: competitive sorption of Cd, Cu, Pb and Zn by three different soils. Soil Water Res 9:97–103
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
.
ESM 1
(PDF 44 kb)
Rights and permissions
About this article
Cite this article
Mouni, L., Belkhiri, L., Bouzaza, A. et al. Interactions between Cd, Cu, Pb, and Zn and four different mine soils. Arab J Geosci 10, 77 (2017). https://doi.org/10.1007/s12517-017-2864-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-2864-9