Abstract
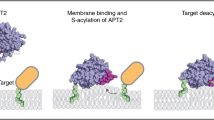
The covalent attachment of lipid moieties to proteins represents an important subclass of co- or post-translational modifications in the cell. The 16-carbon fully saturated lipid palmitate is preferentially conjugated to free cysteines of peripheral or integral membrane proteins. The thioester bond formed can be reversibly hydrolyzed and thereby modulates a proteins function with regard to trafficking, compartmentation within the membrane or lipid-induced conformational changes in a cyclic manner.
Similar content being viewed by others
Literatur
Fukata M, Fukata Y, Adesnik H et al. (2004) Identification of PSD-95 palmitoylating enzymes. Neuron 44:987–996
Rocks O, Gerauer M, Vartak N et al. (2010) The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 141:458–471
Lin DT, Conibear E (2015) ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. Elife 4:e11306
Akimzhanov AM, Boehning D (2015) Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc Natl Acad Sci USA 112:11876–11880
Hundt M, Tabata H, Jeon MS et al. (2006) Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity 24:513–522
Fukata Y, Fukata M (2010) Protein palmitoylation in neuronal development and synaptic plasticity. Nat Rev Neurosci 11:161–175
Montersino A, Thomas GM (2015) Slippery signaling: palmitoylation-dependent control of neuronal kinase localization and activity. Mol Membr Biol 32:179–188
Gottlieb CD, Zhang S, Linder ME (2015) The cysteine-rich domain of the DHHC3 palmitoyltransferase is palmitoylated and contains tightly bound zinc. J Biol Chem 290:29259–29269
Jennings BC, Linder ME (2012) DHHC protein S-acyltransferases use similar ping-pong kinetic mechanisms but display different acyl-CoA specificities. J Biol Chem 287:7236–7245
Korycka J, Lach A, Heger E et al. (2012) Human DHHC proteins: a spotlight on the hidden player of palmitoylation. Eur J Cell Biol 91:107–117
Ji Y, Leymarie N, Haeussler DJ et al. (2013) Direct detection of S-palmitoylation by mass spectrometry. Anal Chem 85:11952–11959
Drisdel RC, Alexander JK, Sayeed A et al. (2006) Assays of protein palmitoylation. Methods 40:127–134.
Martin BR, Cravatt BF (2009) Large-scale profiling of protein palmitoylation in mammalian cells. Nat Methods 6:135–138
Wan J, Roth AF, Bailey AO et al. (2007) Palmitoylated proteins: purification and identification. Nat Protoc 2:1573–1584
Jones ML, Collins MO, Goulding D et al. (2012) Analysis of protein palmitoylation reveals a pervasive role in Plasmodium development and pathogenesis. Cell Host Microbe 12:246–258
Morrison E, Kuropka B, Kliche S et al. (2015) Quantitative analysis of the human T cell palmitome. Sci Rep 5:11598
Greaves J, Chamberlain LH (2011) DHHC palmitoyl transferase: substrate interactions and (patho)physiology. Trends Biochem Sci 36:245–253.
Author information
Authors and Affiliations
Corresponding author
Additional information
Eliot Morrison 2006–2010 Biochemiestudium an der Portland State University, Portland OR, USA. 2010–2012 Biochemie-Masterstudium an der San Francisco State University, San Francisco CA, USA. Seit 2012 Doktorand an der FU Berlin.
Britta Brügger 1988–1994 Biologie- und Biochemiestudium an der Universität Frankfurt a. M. 1994–1998 Promotion am Biochemie-Zentrum der Universität Heidelberg. 1998–2000 Postdoc am Memorial Sloan Kettering Cancer Center New York, NY, USA. 2000–2002 Postdoc am Biochemie-Zentrum der Universität Heidelberg; hier 2002–2014 Akademische Rätin/Oberrätin. Seit 2014 Professorin für Biochemie und Chemische Biologie an der Universität Heidelberg.
Hannah Wiedemann 2009–2015 Chemiestudium an der Universität Heidelberg. Seit 2016 Promotion am Biochemie-Zentrum der Universität Heidelberg.
Christian Freund 1983–1989 Chemiestudium an der Universität Düsseldorf und der LMU München. Hier 1989–1990 Diplomarbeit am Biochemischen Institut. 1990–1994 Promotion am Max-Planck-Institut für Biochemie/LMU München. 1994–1997 Postdoc an der Universität Zürich, Schweiz. 1997–2000 Postdoc an der Harvard Medical School, Boston, MA, USA. 2000–2011 unabhängiger Gruppenleiter am Leibniz-Institut für Molekulare Pharmakologie, Berlin. Seit 2011 Professor für Bio chemie an der FU Berlin.
Rights and permissions
About this article
Cite this article
Morrison, E., Wiedemann, H., Brügger, B. et al. Reversible Palmitoylierung von Proteinen. Biospektrum 23, 32–35 (2017). https://doi.org/10.1007/s12268-017-0763-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12268-017-0763-y