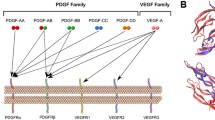
Abstract
Computational modeling of angiogenesis is limited by a lack of experimental data on angiogenic receptor levels. Recent receptor profiling quantified vascular endothelial growth factor receptors (VEGFRs); however data on other angiogenic receptors, such as platelet derived growth factor receptors (PDGFRs), are also necessary for the development of an accurate angiogenesis model. Here, we establish conditions for membrane PDGFR quantification. Additionally, we determine how several environmental conditions control membrane PDGFR levels on human dermal fibroblasts. We demonstrate that membrane PDGFRβ concentrations are negatively correlated with both media serum concentration and cell growth rate, in vitro. We also show VEGF-A165-mediated downregulation of membrane PDGFRα (~25%) and PDGFRβ (~30%), following a 24-h treatment. This supports the idea that VEGF-A165 acts independently of VEGFRs to signal through PDGFRα and PDGFRβ. We observe that PDGF-AA and PDGF-AB downregulate membrane PDGFRα by up to 55 and 75%, respectively, while having little to no effect on PDGFRβ or NRP1. We observe that PDGF-BB effects both PDGFRs and NRP1: membrane PDGFRα and PDGFRβ were downregulated by up to 70 and 90%, respectively, whereas membrane NRP1 was upregulated by up to 40%. These data provide the necessary insight to accurately represent PDGFRs in angiogenesis models, while offering new insight into the regulation of membrane PDGFRs.









Similar content being viewed by others
Abbreviations
- VEGFR:
-
Vascular endothelial growth factor receptor
- PDGFR:
-
Platelet derived growth factor receptor
- NRP:
-
Neuropilin-1
- FGF:
-
Fibroblast growth factor
- TGF:
-
Transforming growth factor
- PAD:
-
Peripheral artery disease
- qFlow:
-
Quantitative flow
- HDF:
-
Human dermal fibroblasts
- HUVEC:
-
Human umbilical vein endothelial cells
- FBS:
-
Fetal bovine serum
- DMSO:
-
Dimethyl sulfoxide
- FGM-2:
-
Fibroblasts growth medium-2
- EGM-2:
-
Endothelial cell growth medium-2
- rhFGF-B:
-
Human recombinant basic fibroblast growth factor
- GA-1000:
-
Gentamicin and amphotericin diluted at a 11000 ratio
- DMEM:
-
Dulbecco’s modified eagle medium
- PES:
-
Polyethersulfone
References
Anderson, S. M., B. Shergill, Z. T. Barry, E. Manousiouthakis, T. T. Chen, E. Botvinick, M. O. Platt, M. L. Iruela-Arispe, and T. Segura. VEGF internalization is not required for VEGFR-2 phosphorylation in bioengineered surfaces with covalently linked VEGF. Integr. Biol. (Camb) 3:887–896, 2011.
Andrae, J., R. Gallini, and C. Betsholtz. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 22:1276–1312, 2008.
Arai, F., A. Hirao, M. Ohmura, H. Sato, S. Matsuoka, K. Takubo, K. Ito, G. Y. Koh, and T. Suda. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118:149–161, 2004.
Augustin, H. G., G. Y. Koh, G. Thurston, and K. Alitalo. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10:165–177, 2009.
Ball, S. G., C. A. Shuttleworth, and C. M. Kielty. Vascular endothelial growth factor can signal through platelet-derived growth factor receptors. J. Cell Biol. 177:489–500, 2007.
Bar, R. S. Interactions of insulin and insulin-like growth factors (IGF) with endothelial cells. Ann. NY Acad. Sci. 401:150–162, 1982.
Barleon, B., S. Sozzani, D. Zhou, H. A. Weich, A. Mantovani, and D. Marmé. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87:3336–3343, 1996.
Battegay, E. J., J. Rupp, L. Iruela-Arispe, E. H. Sage, and M. Pech. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 125:917–928, 1994.
Beitz, J. G., I. S. Kim, P. Calabresi, and A. R. Frackelton. Human microvascular endothelial cells express receptors for platelet-derived growth factor. Proc. Natl. Acad. Sci. USA 88:2021–2025, 1991.
Benjamin, L. E., D. Golijanin, A. Itin, D. Pode, and E. Keshet. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J. Clin. Invest. 103:159–165, 1999.
Benjamin, L. E., I. Hemo, and E. Keshet. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development 125:1591–1598, 1998.
Bentley, K., H. Gerhardt, and P. A. Bates. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initalisation. J. Theor. Biol. 250:25–36, 2008.
Bentley, K., G. Mariggi, H. Gerhardt, and P. A. Bates. Tipping the balance: robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput. Biol. 5:e1000549, 2009.
Bergers, G., S. Song, N. Meyer-Morse, E. Bergsland, and D. Hanahan. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111:1287–1295, 2003.
Betsholtz, C., L. Karlsson, and P. Lindahl. Developmental roles of platelet-derived growth factors. Bioessays 23:494–507, 2001.
Boström, H., K. Willetts, M. Pekny, P. Levéen, P. Lindahl, H. Hedstrand, M. Pekna, M. Hellström, S. Gebre-Medhin, M. Schalling, M. Nilsson, S. Kurland, J. Törnell, J. K. Heath, and C. Betsholtz. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85:863–873, 1996.
Bowen-Pope, D. F., C. E. Hart, and R. A. Seifert. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J. Biol. Chem. 264:2502–2508, 1989.
Brown, L. F., B. Berse, R. W. Jackman, K. Tognazzi, A. J. Guidi, H. F. Dvorak, D. R. Senger, J. L. Connolly, and S. J. Schnitt. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum. Pathol. 26:86–91, 1995.
Brunner, D., J. Frank, H. Appl, H. Schöffl, W. Pfaller, and G. Gstraunthaler. Serum-free cell culture: the serum-free media interactive online database. ALTEX 27:53–62, 2010.
Burrell, R. A., N. McGranahan, J. Bartek, and C. Swanton. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501:338–345, 2013.
Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 6:389–395, 2000.
Carmeliet, P., and D. Collen. Vascular development and disorders: molecular analysis and pathogenic insights. Kidney Int. 53:1519–1549, 1998.
Casanovas, O., D. J. Hicklin, G. Bergers, and D. Hanahan. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8:299–309, 2005.
Chen, S., P. Gupta, G. Conard, J. C. Weddell, J. Parkin, W. Woods, and P. I. Imoukhuede. Towards clinical application: Establishing in vitro standards for qFlow cytometry-based profiling of receptors In Revision.
Cirit, M., and J. M. Haugh. Data-driven modelling of receptor tyrosine kinase signalling networks quantifies receptor-specific potencies of PI3 K- and Ras-dependent ERK activation. Biochem. J. 441:77–85, 2012.
Claesson-Welsh, L. Signal transduction by the PDGF receptors. Prog. Growth Factor Res. 5:37–54, 1994.
Dejana, E. Endothelial cell-cell junctions: happy together. Nat. Rev. Mol. Cell Biol. 5:261–270, 2004.
Dong, J., J. Grunstein, M. Tejada, F. Peale, G. Frantz, W.-C. Liang, W. Bai, L. Yu, J. Kowalski, X. Liang, G. Fuh, H.-P. Gerber, and N. Ferrara. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 23:2800–2810, 2004.
Donovan, J., X. Shiwen, J. Norman, and D. Abraham. Platelet-derived growth factor alpha and beta receptors have overlapping functional activities towards fibroblasts. Fibrogenesis Tissue Repair 6:10, 2013.
Ejiri, H., T. Nomura, M. Hasegawa, C. Tatsumi, M. Imai, S. Sakakibara, and H. Terashi. Use of synthetic serum-free medium for culture of human dermal fibroblasts to establish an experimental system similar to living dermis. Cytotechnology 67(3):507–514, 2014.
Erber, R., A. Thurnher, A. D. Katsen, G. Groth, H. Kerger, H.-P. Hammes, M. D. Menger, A. Ullrich, and P. Vajkoczy. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 18:338–340, 2004.
Escobedo, J. A., S. Navankasatussas, L. S. Cousens, S. R. Coughlin, G. I. Bell, and L. T. Williams. A common PDGF receptor is activated by homodimeric A and B forms of PDGF. Science 240:1532–1534, 1988.
Fantin, A., J. M. Vieira, G. Gestri, L. Denti, Q. Schwarz, S. Prykhozhij, F. Peri, S. W. Wilson, and C. Ruhrberg. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116:829–840, 2010.
Finley, S. D., L.-H. Chu, and A. S. Popel. Computational systems biology approaches to anti-angiogenic cancer therapeutics. Today: Drug Discov., 2014; (00).
Finley, S. D., M. O. Engel-Stefanini, P. I. Imoukhuede, and A. S. Popel. Pharmacokinetics and pharmacodynamics of VEGF-neutralizing antibodies. BMC Syst Biol 5(1):193, 2011.
Forsberg, K., I. Valyi-Nagy, C. H. Heldin, M. Herlyn, and B. Westermark. Platelet-derived growth factor (PDGF) in oncogenesis: development of a vascular connective tissue stroma in xenotransplanted human melanoma producing PDGF-BB. Proc. Natl. Acad. Sci. 90:393–397, 1993.
Fredriksson, L., H. Li, and U. Eriksson. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 15:197–204, 2004.
Gabhann, F. Mac, and A. S. Popel. Systems biology of vascular endothelial growth factors. Microcirculation 15:715–738, 2008.
Gough, A. H., N. Chen, T. Y. Shun, T. R. Lezon, R. C. Boltz, C. E. Reese, J. Wagner, L. A. Vernetti, J. R. Grandis, A. V. Lee, A. M. Stern, M. E. Schurdak, and D. L. Taylor. Identifying and quantifying heterogeneity in high content analysis: application of heterogeneity indices to drug discovery. PLoS One 9:e102678, 2014.
Greenberg, J. I., and D. A. Cheresh. VEGF as an inhibitor of tumor vessel maturation: implications for cancer therapy. Expert Opin. Biol. Ther. 9:1347–1356, 2009.
Greenberg, J. I., D. J. Shields, S. G. Barillas, L. M. Acevedo, E. Murphy, J. Huang, L. Scheppke, C. Stockmann, R. S. Johnson, N. Angle, and D. A. Cheresh. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456:809–813, 2008.
Greenhalgh, D. G., K. H. Sprugel, M. J. Murray, and R. Ross. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 136:1235–1246, 1990.
Guaiquil, V. H., S. Swendeman, W. Zhou, P. Guaiquil, G. Weskamp, J. W. Bartsch, and C. P. Blobel. ADAM8 is a negative regulator of retinal neovascularization and of the growth of heterotopically injected tumor cells in mice. J. Mol. Med. (Berl) 88:497–505, 2010.
Hammacher, A., K. Mellström, C. H. Heldin, and B. Westermark. Isoform-specific induction of actin reorganization by platelet-derived growth factor suggests that the functionally active receptor is a dimer. EMBO J. 8:2489–2495, 1989.
Hanahan, D., and R. A. Weinberg. Hallmarks of cancer: the next generation. Cell 144:646–674, 2011.
Hart, C. E., J. W. Forstrom, J. D. Kelly, R. A. Seifert, R. A. Smith, R. Ross, M. J. Murray, and D. F. Bowen-Pope. Two classes of PDGF receptor recognize different isoforms of PDGF. Science 240:1529–1531, 1988.
He, B., R. Baird, R. Butera, A. Datta, S. George, B. Hecht, A. Hero, G. Lazzi, R. C. Lee, J. Liang, M. Neuman, G. C. Y. Peng, E. J. Perreault, M. Ramasubramanian, M. D. Wang, J. Wikswo, G.-Z. Yang, and Y.-T. Zhang. Grand challenges in interfacing engineering with life sciences and medicine. IEEE Trans. Biomed. Eng. 60:589–598, 2013.
Heldin, C.-H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 11:97, 2013.
Heldin, C.-H., A. Östman, and L. Rönnstrand. Signal transduction via platelet-derived growth factor receptors. Biochim. Biophys. Acta 1378:F79–F113, 1998.
Heldin, C. H., A. Wasteson, and B. Westermark. Platelet-derived growth factor. Mol. Cell. Endocrinol. 39:169–187, 1985.
Heldin, C. H., and B. Westermark. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1:555–566, 1990.
Heldin, C. H., and B. Westermark. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79:1283–1316, 1999.
Heldin, C. H., B. Westermark, and A. Wasteson. Specific receptors for platelet-derived growth factor on cells derived from connective tissue and glia. Proc. Natl. Acad. Sci. USA 78:3664–3668, 1981.
Ichiki, Y., E. Smith, E. C. LeRoy, and M. Trojanowska. Different effects of basic fibroblast growth factor and transforming growth factor-beta on the two platelet-derived growth factor receptors’ expression in scleroderma and healthy human dermal fibroblasts. J. Invest. Dermatol. 104:124–127, 1995.
Imoukhuede, P. I., A. O. Dokun, B. H. Annex, and A. S. Popel. Endothelial cell-by-cell profiling reveals the temporal dynamics of VEGFR1 and VEGFR2 membrane localization after murine hindlimb ischemia. Am. J. Physiol. Heart. Circ. Physiol. 304:H1085–H1093, 2013.
Imoukhuede, P. I., and A. S. Popel. Quantification and cell-to-cell variation of vascular endothelial growth factor receptors. Exp. Cell Res. 317:955–965, 2011.
Imoukhuede, P. I., and A. S. Popel. Expression of VEGF receptors on endothelial cells in mouse skeletal muscle. PLoS One 7:e44791, 2012.
Imoukhuede, P. I., and A. S. Popel. Quantitative fluorescent profiling of VEGFRs reveals tumor cell and endothelial cell heterogeneity in breast cancer xenografts. Cancer Med. 3:225–244, 2014.
Jakobsson, L., C. A. Franco, K. Bentley, R. T. Collins, B. Ponsioen, I. M. Aspalter, I. Rosewell, M. Busse, G. Thurston, A. Medvinsky, S. Schulte-Merker, and H. Gerhardt. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 12:943–953, 2010.
Ji, J. W., F. Mac Gabhann, and A. S. Popel. Skeletal muscle VEGF gradients in peripheral arterial disease: simulations of rest and exercise. Am. J. Physiol. Heart Circ. Physiol. 293:H3740–H3749, 2007.
Keegan, P. M., C. L. Wilder, and M. O. Platt. Tumor necrosis factor alpha stimulates cathepsin K and V activity via juxtacrine monocyte-endothelial cell signaling and JNK activation. Mol. Cell. Biochem. 367:65–72, 2012.
Kut, C., F. Mac Gabhann, and A. S. Popel. Where is VEGF in the body? A meta-analysis of VEGF distribution in cancer. Br. J. Cancer 97:978–985, 2007.
Levéen, P., M. Pekny, S. Gebre-Medhin, B. Swolin, E. Larsson, and C. Betsholtz. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 8:1875–1887, 1994.
Li, X., A. Pontén, K. Aase, L. Karlsson, A. Abramsson, M. Uutela, G. Bäckström, M. Hellström, H. Boström, H. Li, P. Soriano, C. Betsholtz, C. H. Heldin, K. Alitalo, A. Ostman, and U. Eriksson. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat. Cell Biol. 2:302–309, 2000.
Lieu, C., J. Heymach, M. Overman, H. Tran, and S. Kopetz. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin. Cancer Res. 17:6130–6139, 2011.
Lin, S.-L., F.-C. Chang, C. Schrimpf, Y.-T. Chen, C.-F. Wu, V.-C. Wu, W.-C. Chiang, F. Kuhnert, C. J. Kuo, Y.-M. Chen, K.-D. Wu, T.-J. Tsai, and J. S. Duffield. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am. J. Pathol. 178:911–923, 2011.
Lindner, V., and M. A. Reidy. Platelet-derived growth factor ligand and receptor expression by large vessel endothelium in vivo. Am. J. Pathol. 146:1488–1497, 1995.
Liu, G., A. A. Qutub, P. Vempati, F. Mac Gabhann, and A. S. Popel. Module-based multiscale simulation of angiogenesis in skeletal muscle. Theor. Biol. Med. Model. 8:6, 2011.
Lokker, N. A., J. P. O’Hare, A. Barsoumian, J. E. Tomlinson, V. Ramakrishnan, L. J. Fretto, and N. A. Giese. Functional importance of platelet-derived growth factor (PDGF) receptor extracellular immunoglobulin-like domains. Identification of PDGF binding site and neutralizing monoclonal antibodies. J. Biol. Chem. 272:33037–33044, 1997.
Lynch, S. E., J. C. Nixon, R. B. Colvin, and H. N. Antoniades. Role of platelet-derived growth factor in wound healing: synergistic effects with other growth factors. Proc. Natl. Acad. Sci. 84:7696–7700, 1987.
Mac Gabhann, F., J. W. Ji, and A. S. Popel. VEGF gradients, receptor activation, and sprout guidance in resting and exercising skeletal muscle. J. Appl. Physiol. 102:722–734, 2007.
Mac Gabhann, F., A. A. Qutub, B. H. Annex, and A. S. Popel. Systems biology of pro-angiogenic therapies targeting the VEGF system. Wiley Interdiscip. Rev. 2:694–707, 2010.
Mamer, S. B., M. Kumar, and P. I. Imoukhuede. Novel VEGF-PDGF cross-family binding kinetics revealed through optimized surface plasmon resonance-based assay. In Review*.
Matsui, T., J. H. Pierce, T. P. Fleming, J. S. Greenberger, W. J. LaRochelle, M. Ruggiero, and S. A. Aaronson. Independent expression of human alpha or beta platelet-derived growth factor receptor cDNAs in a naive hematopoietic cell leads to functional coupling with mitogenic and chemotactic signaling pathways. Proc. Natl. Acad. Sci. USA 86:8314–8318, 1989.
Micke, P., and A. Ostman. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer 45(Suppl 2):S163–S175, 2004.
Morikawa, S., P. Baluk, T. Kaidoh, A. Haskell, R. K. Jain, and D. M. McDonald. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 160:985–1000, 2002.
Napione, L., S. Pavan, A. Veglio, A. Picco, G. Boffetta, A. Celani, G. Seano, L. Primo, A. Gamba, and F. Bussolino. Unraveling the influence of endothelial cell density on VEGF-A signaling. Blood 119:5599–5607, 2012.
Neufeld, G., T. Cohen, S. Gengrinovitch, and Z. Poltorak. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13:9–22, 1999.
Ostman, A., and C. H. Heldin. Involvement of platelet-derived growth factor in disease: development of specific antagonists. Adv. Cancer Res. 80:1–38, 2001.
Pannu, K. K., E. T. Joe, and S. B. Iyer. Performance evaluation of quantiBRITE phycoerythrin beads. Cytometry 45:250–258, 2001.
Park, C. S., I. C. Schneider, and J. M. Haugh. Kinetic analysis of platelet-derived growth factor receptor/phosphoinositide 3-kinase/Akt signaling in fibroblasts. J. Biol. Chem. 278:37064–37072, 2003.
Paulsson, J., T. Sjöblom, P. Micke, F. Pontén, G. Landberg, C.-H. Heldin, J. Bergh, D. J. Brennan, K. Jirström, and A. Ostman. Prognostic significance of stromal platelet-derived growth factor beta-receptor expression in human breast cancer. Am. J. Pathol. 175:334–341, 2009.
Pennock, S., and A. Kazlauskas. Vascular endothelial growth factor A competitively inhibits platelet-derived growth factor (PDGF)-dependent activation of PDGF receptor and subsequent signaling events and cellular responses. Mol. Cell. Biol. 32:1955–1966, 2012.
Pierce, G. F., T. A. Mustoe, B. W. Altrock, T. F. Deuel, and A. Thomason. Role of platelet-derived growth factor in wound healing. J. Cell. Biochem. 45:319–365, 1991.
Qutub, A. A., F. Mac Gabhann, E. D. Karagiannis, P. Vempati, and A. S. Popel. Multiscale models of angiogenesis. IEEE Eng. Med. Biol. Mag. 28:14–31, 2009.
Qutub, A. A., and A. S. Popel. Elongation, proliferation & migration differentiate endothelial cell phenotypes and determine capillary sprouting. BMC Syst. Biol. 3:13, 2009.
Räsänen, K., and A. Vaheri. Activation of fibroblasts in cancer stroma. Exp. Cell Res. 316:2713–2722, 2010.
Renner, O., A. Tsimpas, S. Kostin, S. Valable, E. Petit, W. Schaper, and H. H. Marti. Time- and cell type-specific induction of platelet-derived growth factor receptor-β during cerebral ischemia. Mol. Brain Res. 113:44–51, 2003.
Robson, M. C., L. G. Phillips, A. Thomason, L. E. Robson, and G. F. Pierce. Platelet-derived growth factor BB for the treatment of chronic pressure ulcers. Lancet 339:23–25, 1992.
Rosenkranz, S., and A. Kazlauskas. Evidence for distinct signaling properties and biological responses induced by the PDGF receptor alpha and beta subtypes. Growth Factors 16:201–216, 1999.
Roxworthy, B. J., M. T. Johnston, F. T. Lee-Montiel, R. H. Ewoldt, P. I. Imoukhuede, and K. C. Toussaint. Plasmonic optical trapping in biologically relevant media. PLoS One 9:e93929, 2014.
Scharpfenecker, M., U. Fiedler, Y. Reiss, and H. G. Augustin. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell Sci. 118:771–780, 2005.
Seifert, R. A., C. E. Hart, P. E. Phillips, J. W. Forstrom, R. Ross, M. J. Murray, and D. F. Bowen-Pope. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J. Biol. Chem. 264:8771–8778, 1989.
Severinsson, L., L. Claesson-Welsh, and C. H. Heldin. A B-type PDGF receptor lacking most of the intracellular domain escapes degradation after ligand binding. Eur. J. Biochem. 182:679–686, 1989.
Shen, W., C. Zhang, M. W. Fannon, K. Forsten-Williams, and J. Zhang. A computational model of FGF-2 binding and HSPG regulation under flow. IEEE Trans. Biomed. Eng. 56:2147–2155, 2009.
Shigematsu, S., K. Yamauchi, K. Nakajima, S. Iijima, T. Aizawa, and K. Hashizume. IGF-1 regulates migration and angiogenesis of human endothelial cells. Endocr. J. 46(Suppl):S59–S62, 1999.
Shweiki, D., A. Itin, D. Soffer, and E. Keshet. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845, 1992.
Simm, A., M. Nestler, and V. Hoppe. PDGF-AA, a potent mitogen for cardiac fibroblasts from adult rats. J. Mol. Cell. Cardiol. 29:357–368, 1997.
Sorkin, A., B. Westermark, C. H. Heldin, and L. Claesson-Welsh. Effect of receptor kinase inactivation on the rate of internalization and degradation of PDGF and the PDGF beta-receptor. J. Cell Biol. 112:469–478, 1991.
Sugimoto, H., T. M. Mundel, M. W. Kieran, and R. Kalluri. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 5:1640–1646, 2006.
Takahashi, Y., Y. Kitadai, C. D. Bucana, K. R. Cleary, and L. M. Ellis. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 55:3964–3968, 1995.
Thurston, G., J. S. Rudge, E. Ioffe, H. Zhou, L. Ross, S. D. Croll, N. Glazer, J. Holash, D. M. McDonald, and G. D. Yancopoulos. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 6:460–463, 2000.
Tsirakis, G., C. A. Pappa, P. Kanellou, M. A. Stratinaki, A. Xekalou, F. E. Psarakis, G. Sakellaris, A. Alegakis, E. N. Stathopoulos, and M. G. Alexandrakis. Role of platelet-derived growth factor-AB in tumour growth and angiogenesis in relation with other angiogenic cytokines in multiple myeloma. Hematol. Oncol. 30:131–136, 2012.
Vempati, P., F. Mac Gabhann, and A. S. Popel. Quantifying the proteolytic release of extracellular matrix-sequestered VEGF with a computational model. PLoS One 5:e11860, 2010.
Vempati, P., A. S. Popel, and F. Mac Gabhann. Formation of VEGF isoform-specific spatial distributions governing angiogenesis: computational analysis. BMC Syst. Biol. 5:59, 2011.
Vempati, P., A. S. Popel, and F. Mac Gabhann. Extracellular regulation of VEGF: isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 25:1–19, 2014.
Von Tell, D., A. Armulik, and C. Betsholtz. Pericytes and vascular stability. Exp. Cell Res. 312:623–629, 2006.
Wang, L., F. Abbasi, A. K. Gaigalas, R. F. Vogt, and G. E. Marti. Discrepancy in measuring CD4 expression on T-lymphocytes using fluorescein conjugates in comparison with unimolar CD4-phycoerythrin conjugates. Cytometry B. Clin. Cytom. 70:410–415, 2006.
Weddell, J. C., and P. I. Imoukhuede. Quantitative characterization of cellular membrane-receptor heterogeneity through statistical and computational modeling. PLoS One 9:e97271, 2014.
Weskamp, G., K. Mendelson, S. Swendeman, S. Le Gall, Y. Ma, S. Lyman, A. Hinoki, S. Eguchi, V. Guaiquil, K. Horiuchi, and C. P. Blobel. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ. Res. 106:932–940, 2010.
Willett, C. G., Y. Boucher, E. di Tomaso, D. G. Duda, L. L. Munn, R. T. Tong, D. C. Chung, D. V. Sahani, S. P. Kalva, S. V. Kozin, M. Mino, K. S. Cohen, D. T. Scadden, A. C. Hartford, A. J. Fischman, J. W. Clark, D. P. Ryan, A. X. Zhu, L. S. Blaszkowsky, H. X. Chen, P. C. Shellito, G. Y. Lauwers, and R. K. Jain. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 10:145–147, 2004.
Yamazaki, Y., and T. Morita. Molecular and functional diversity of vascular endothelial growth factors. Mol. Divers. 10:515–527, 2006.
Yoshida, A., B. Anand-Apte, and B. R. Zetter. Differential endothelial migration and proliferation to basic fibroblast growth factor and vascular endothelial growth factor. Growth Factors 13:57–64, 1996.
Zhang, J., R. Cao, Y. Zhang, T. Jia, Y. Cao, and E. Wahlberg. Differential roles of PDGFR-alpha and PDGFR-beta in angiogenesis and vessel stability. FASEB J. 23:153–163, 2009.
Acknowledgments
We would like to thank the American Cancer Society, Illinois Division Basic Research Grant, as well as the National Cancer Institute Grant for funding support. We would like to thank Audra Storm, Brendan Mathias, and Dipen Kumar for assistance with experiments, and Jared Weddell, Spencer Mamer, and Ali Ansari for insightful discussion. We also thank Marinos Kalafatis for careful and critical reading of the manuscript.
Conflict of interest
Si Chen, Xinyi Guo, Osazomon Imarenezor and PI Imoukhuede declare that they have no conflicts of interest.
Ethical Standards
No human studies or animal studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
This article is part of the 2015 Young Innovators Issue.
P.I. Imoukhuede Assistant Professor Imoukhuede is a native of Illinois, having attended Rich South High School and the Illinois Mathematics and Science Academy (IMSA). Assistant Professor Imoukhuede earned her SB in Chemical Engineering from the Massachusetts Institute of Technology (MIT) where her research earned her the coveted Class of 1972 award, presented annually to the project that most improves the quality of life through its impact on people and/or the environment. Assistant Professor Imoukhuede’s research was funded by the National Science Foundation’s Biotechnology Process Engineering Center at MIT and through a Bioengineering Undergraduate Research Award by the MIT Division of Bioengineering and Environmental Health. Assistant Professor Imoukhuede was also an NCAA All-American athlete, garnering these honors three times for placing at the NCAA Track and Field Championships. Assistant Professor Imoukhuede was honored with the 2002 Betsy Schumaker Award (also known as the MIT female athlete of the year), was selected to a COSIDA/VERIZON Academic All-America team, and was awarded an NCAA postgraduate scholarship. Assistant Professor Imoukhuede championed the importance of social responsibility in the midst of academic excellence by serving as the President of the MIT Committee on Multiculturalism, President of the MIT chapter of the American Institute of Chemical Engineers (AIChE), and held both chapter and zone offices in the National Society of Black Engineers (NSBE). After earning her undergraduate degree, Assistant Professor Imoukhuede pursued graduate study in Bioengineering at the California Institute of Technology (Caltech) in Pasadena, CA. Here, she combined sensitive techniques in biomedical optics with nanoparticle imaging towards understanding the structure, function, and trafficking of a key protein in epilepsy, the GABA transporter, GAT1. She also performed research in nicotine addiction through molecular imaging of nicotinic acetylcholine receptors. Assistant Professor Imoukhuede’s research in nanotechnology earned her the Kavli Nanoscience Institute Award and her graduate research was supported by the National Institutes of Health (NIDA). Assistant Professor Imoukhuede was the first African-American woman to be awarded a Bioengineering PhD by Caltech and was only the second African-American woman to earn a PhD from Caltech’s Division of Engineering and Applied Science. Assistant Professor Imoukhuede completed a Postdoctoral Fellowship in the Biomedical Engineering Department at the Johns Hopkins University School of Medicine. During her fellowship at Johns Hopkins, she was 1 of 10 postdoctoral fellows nationwide to earn the prestigious United Negro College Fund/Merck Postdoctoral Research Fellowship, 1 of 6 young investigators to earn the FASEB Postdoctoral Professional Development Award, and her work was awarded a Poster Award at the biennial Gordon Conference in Angiogenesis. Her postdoctoral work was also supported by the National Institutes of Health (NHLBI). Currently, Assistant Professor Princess Imoukhuede aims to advance our cellular and molecular understanding of cancer regulation through systems biology. Assistant Professor Imoukhuede has extensive training in bioengineering and biophysics; as such, her laboratory leads efforts to sense, model, predict, and ultimately tune tumor angiogenesis by developing novel tumor cell isolation platforms, mapping tumor heterogeneity, and integrating these tumor defining parameters through computational modeling. Towards these goals, Prof. Imoukhuede is currently developing novel approaches that can quickly, and efficiently isolate specific cell types without disrupting cell-surface receptor-levels. Prof. Imoukhuede has recently pioneered a novel quantitative fluorescence approach for sensitive cell isolation and mapping of angiogenic receptor surface-distributions. She has applied this technology to animal models of both breast cancer and ischemic disease. Prof. Imoukhuede incorporates these molecular and cellular data into multi-scale computational models. Her models have recently predicted the efficacy of anti-angiogenic therapeutics, predicted optimal anti-angiogenic therapeutic parameters, and predicted the effect of tumor heterogeneity on anti-angiogenic therapeutics. Her advancement of this experimental (cell isolation and profiling) and computational, paradigm accelerates discovery into the signaling cues mediating tumor growth and development. Assistant Professor Imoukhuede’s biography is featured in the book, A Hand Up: Women Mentoring Women in Science.

Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, S., Guo, X., Imarenezor, O. et al. Quantification of VEGFRs, NRP1, and PDGFRs on Endothelial Cells and Fibroblasts Reveals Serum, Intra-Family Ligand, and Cross-Family Ligand Regulation. Cel. Mol. Bioeng. 8, 383–403 (2015). https://doi.org/10.1007/s12195-015-0411-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-015-0411-x