Abstract
Purpose
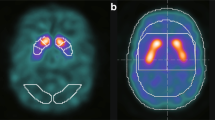
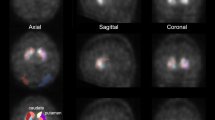
Quantification of the tracer distribution would add objectivity to the visual assessments of dopamine transporter (DAT) single photon emission computed tomography (SPECT) data. Our study aimed to evaluate the diagnostic utility of fractal dimension (FD) as a quantitative indicator of tracer distribution and compared with the conventional quantitative value: specific binding ratio (SBR). We also evaluated the utility of the combined index SBR/FD (SBR divided by FD).
Materials and methods
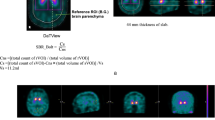
We conducted both clinical and phantom studies. In the clinical study, 150 patients including 110 patients with Parkinsonian syndrome (PS) and 40 without PS were enrolled. In the phantom study, we used a striatal phantom with the striatum chamber divided into two spaces, representing the caudate nucleus and putamen. The SBR, FD, and SBR/FD were calculated and compared between datasets for evaluating the diagnostic utility. Mann–Whitney test and receiver-operating characteristics (ROC) analysis were used for analysis.
Results
ROC analysis revealed that the FD value had high diagnostic performance [the areas under the curve (AUC) = 0.943] and the combined use of SBR and FD (SBR/FD) delivered better results than the SBR alone (AUC, 0.964 vs 0.899; p < 0.001). The sensitivity, specificity, and accuracy, respectively, were 79.1, 85.0, and 80.7% with SBR, 84.5, 97.5, and 88.0% with FD, and 92.7, 87.5, and 91.3% with SBR/FD.
Conclusion
Our results confirmed that the FD value is a useful diagnostic index, which reflects the tracer distribution in DAT SPECT images. The combined use of SBR and FD was more useful than either used alone.







Similar content being viewed by others
Abbreviations
- PD:
-
Parkinson’s disease
- PET:
-
Positron emission tomography
- SPECT:
-
Single photon emission computed tomography
- DAT:
-
Dopamine transporter
- PS:
-
Parkinsonian syndrome
- DLB:
-
Dementia with Lewy body
- SBR:
-
Specific binding ratio
- VOI:
-
Volume of interest
- FD:
-
Fractal dimension
- 3D-FA:
-
Three-dimensional-fractal analysis
- NPS:
-
Non Parkinsonian syndrome
- RI:
-
Radioisotope
- OSEM:
-
Ordered-subset expectation maximization
- ICC:
-
Intra-class correlation coefficient
- ROC:
-
Receiver-operating characteristics
- AUC:
-
Area under the ROC curve
- LOA:
-
Limit of agreement
- SSRI:
-
Selective serotonin reuptake inhibitor
References
Vlaar AM, van Kroonenburgh MJ, Kessels AG, Weber WE. Meta-analysis of the literature on diagnostic accuracy of SPECT in parkinsonian syndromes. BMC Neurol. 2007;7:27.
McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–13.
Kwak Y, Müller ML, Bohnen NI, Dayalu P, Seidler RD. Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson’s disease. J Neurophysiol. 2010;103:942–9.
Tondeur MC, Hambye AS, Dethy S, Ham HR. Interobserver reproducibility of the interpretation of I-123 FP-CIT single-photon emission computed tomography. Nucl Med Commun. 2010;31:717–25.
Tossici-Bolt L, Hoffmann SM, Kemp PM, Mehta RL, Fleming JS. Quantification of [123I]FP-CIT SPECT brain images: an accurate technique for measurement of the specific binding ratio. Eur J Nucl Med Mol Imaging. 2006;33:1491–9.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I] FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40:213–27.
Nonokuma M, Kuwabara Y, Hida K, Tani T, Takano K, Yoshimitsu K. Optimal ROI setting on the anatomically normalized I-123 FP-CIT images using high-resolution SPECT. Ann Nucl Med. 2016;30:637–44.
Nagao M, Murase K, Yasuhara Y, Ikezoe J. Quantitative analysis of pulmonary emphysema: three-dimensional fractal analysis of single-photon emission computed tomography images obtained with a carbon particle radioaerosol. Am J Roentgenol. 1998;171:1657–63.
Nagao M, Murase K, Ichiki T, Sakai S, Yasuhara Y, Ikezoe J. Quantitative analysis of technegas SPECT: evaluation of regional severity of emphysema. J Nucl Med. 2000;41:590–5.
Nagao M, Murase K. Measurement of heterogeneous distribution on Technegas SPECT images by three-dimensional fractal analysis. Ann Nucl Med. 2002;16:369–76.
Nagao M, Murase K, Kikuchi T, Ikeda M, Nebu A, Fukuhara R, et al. Fractal analysis of cerebral blood flow distribution in Alzheimer’s disease. J Nucl Med. 2001;42:1446–50.
Nagao M, Sugawara Y, Ikeda M, Fukuhara R, Hokoishi K, Murase K, et al. Heterogeneity of cerebral blood flow in frontotemporal lobar degeneration and Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2004;31:162–8.
Nagao M, Sugawara Y, Ikeda M, et al. Heterogeneity of posterior limbic perfusion in very early Alzheimer’s disease. Neurosci Res. 2006;55:285–91.
Södoferlund TA, Dickson JC, Prvulovich E, Ben-Haim S, Kemp P, Booij J, et al. Value of semiquantitative analysis for clinical reporting of 123I-2-β-carbomethoxy-3β-(4-iodopenyl)-N-(3-fluoropropyl)nortropane SPECT studies. J Nucl Med. 2013;54:714–22.
Mäkinen E, Joutsa J, Johansson J, Mäki M, Seppänen M, Kaasinen V. Visual versus automated analysis of [I-123]FP-CIT SPECT scans in parkinsonism. J Neural Transm (Vienna). 2016;123:1309–18.
Booij J, Dubroff J, Pryma D, Yu J, Agarwal R, Lakhani P, et al. Diagnostic performance of the visual reading of 123I-Ioflupane SPECT images when assessed with or without quantification in patients with movement disorders or dementia. J Nucl Med 2017. https://doi.org/10.2967/jnumed.116.189266 (Epub ahead of print).
Nicastro N, Garibotto V, Allali G, Assal F, Burkhard PR. Added value of combined semi-quantitative and visual [123I]FP-CIT SPECT analyses for the diagnosis of dementia with Lewy Bodies. Clin Nucl Med. 2017;42(2):e96–e102.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Michallek F, Dewey M. Fractal analysis in radiological and nuclear medicine perfusion imaging: a systematic review. Eur Radiol. 2014;24:60–9.
Kuikka JT, Tiihonen J, Karhu J, Bergström KA, Räsänen P. Fractal analysis of striatal dopamine re-uptake sites. Eur J Nucl Med. 1997;24:1085–90.
Kuikka JT, Yang J, Karhu J, Laitinen T, Tupala E, Hallikainen T, et al. Imaging the structure of the striatum: a fractal approach to SPECT image interpretation. Physiol Meas. 1998;19:367–74.
Kuikka JT, Tiihonen J, Bergström KA, Karhu J, Räsänen P, Eronen M. Abnormal structure of human striatal dopamine re-uptake sites in habitually violent alcoholic offenders: a fractal analysis. Neurosci Lett. 1998;253:195–7.
Bolt L, Fleming JS, Kemp PM. The 3D fractal dimension of DaTSCAN Images. Nucl Med Commun. 2006;27:296 (abstract).
Bolt L, Fleming JS, Kemp PM. Quantifying DaTSCAN TM images- a comparison of region-of-interest and fractal analysis. Eur J Nucl Med Mol Imaging. 2006;33:S98 (abstract).
Yokoyama K, Imabayashi E, Sumida K, Sone D, Kimura Y, Sato N, et al. Computed-tomography-guided anatomic standardization for quantitative assessment of dopamine transporter SPECT. Eur J Nucl Med Mol Imaging. 2017;44:366–72.
Kim JS, Cho H, Choi JY, Lee SH, Ryu YH, Lyoo CH, et al. Feasibility of computed tomography-guided methods for spatial normalization of dopamine transporter positron emission tomography image. PLoS One. 2015;10(7):e0132585. https://doi.org/10.1371/journal.pone.0132585.
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86:566 – 76.
Dickson JC, Tossici-Bolt L, Sera T, Booij J, Ziebell M, Morbelli S, et al. The impact of reconstruction and scanner characterisation on the diagnostic capability of a normal database for [123I]FP-CIT SPECT imaging. EJNMMI Res. 2017;7:10. https://doi.org/10.1186/s13550-016-0253-0 (Epub 2017 Jan 24).
Acknowledgements
The authors thank the staff of the Division of Nuclear Medicine at the Department of Diagnostic Radiology, for their valuable support. We also thank Editage for English language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Iwabuchi, Y., Nakahara, T., Kameyama, M. et al. Quantitative evaluation of the tracer distribution in dopamine transporter SPECT for objective interpretation. Ann Nucl Med 32, 363–371 (2018). https://doi.org/10.1007/s12149-018-1256-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-018-1256-x