Abstract
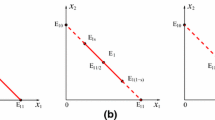
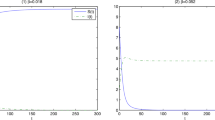
Bacteriophage are ubiquitous in nature, yet many central aspects of host–phage biology have not been integrated into mathematical models. We propose a novel model of host–phage population dynamics that accounts for the decreased ability of phages to lyse hosts as hosts approach their carrying capacity. In contrast to existing predator–prey-like models, we find a parameter regime in which phages cannot invade a host-only system but, nonetheless, can stably coexist with hosts at lower densities. The finding of alternative stable states suggests clear linkages with observed life history strategies of phages. In addition, we solve a limiting case of the proposed model and show that conservative predator-prey like systems do not inevitably exhibit population cycles. Finally, we discuss possible extensions of the present model and scenarios for experimental testing.





Similar content being viewed by others
References
Abedon ST, Yin J (2006) Bacteriophage plaques: theory and analysis. In: Kropinski M (ed) Bacteriophages: methods and protocols. Humana Press, Totowa, NJ
Beretta E, Kuang Y (2001) Modeling and analysis of a marine bacteriophage infection with latency period. Nonlinear Anal Real World Appl 2:35–74
Bohannan BJM, Lenski RE (1997) Effect of resource enrichment on a chemostat community of bacteria and bacteriophage. Ecology 78:2303–2315
Breitbart M, Salamon P, Andresen B, Mahaffy JM, Segall AM, Mead D, Azam F, Rohwer, F (2002) Genomic analysis of uncultured marine viral communities. Proc Natl Acad Sci USA 99:14250–14255
Bull JJ, Millstein J, Orcutt J, Wichman HA (2006) Evolutionary feedback mediated through population density, illustrated with viruses in chemostats. Am Nat 167:E39–E51
Burch CL, Chao L (2004) Epistasis and its relationship to canalization in the RNA virus ϕ6. Genetics 167:559–567
Chao L, Levin BR, Stewart FM (1977) A complex community in a simple habitat: an experimental study with bacteria and phage. Ecology 58:369–378
Cohen SS (1949) Growth requirements of bacterial viruses. Bacteriol Rev 13:1–24
Culley AI, Chan AM, Suttle CA (2006) Metagenomic analysis of coastal RNA virus communities. Science 312:1795–1798
De Paepe M, Taddei F (2006) Viruses’ life history: towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biol 4:e193
Edwards RA, Rohwer F (2005) Viral metagenomics. Nat Rev Microbiol 3:504–510
Faruque SM, Islam MJ, Ahmad QA, Faruque ASG, Sack DA, Nair GB, Mekalanos JJ (2005a) Self-limiting nature of aseasonal cholera epidemics: Role of host-mediated amplification of phage. Proc Natl Acad Sci USA 102:6119–6124
Faruque SM, Naser IB, Islam MJ, Faruque ASG, Ghosh AN, Nair GB, Sack DA, Mekalanos JJ (2005b) Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci USA 102:1702–1707
Forde SE, Thompson JN, Bohannan BJM (2004) Adaptation varies through space and time in a coevoling host–parasitoid interaction. Nature 431:841–844
Fort J, Méndez V (2002) Time-delayed spread of viruses in growing plaques. Phys Rev Lett 89:178101
Fuhrman JA (1999) Marine viruses and their biogeochemical and ecological effects. Nature 399:541–548
Haywood AM (1974) Lysis of RNA phage-infected cells depends upon culture conditions. J Gen Virol 22:431–435
Lenski RE (1988) Dynamics of interactions between bacteria and virulent bacteriophage. Adv Microb Ecol 10:1–44
Levin BR, Stewart FM, Chao L (1977) Resource-limited growth, competition, and predation: a model and experimental studies with bacteria and bacteriophage. Am Nat 111:3–24
Lindell DL, Sullivan MB, Johnson ZI, Tolonen A, Rohwer F, Chisholm SW (2004) Transfer of photosnthetic genes to and from Prochlorococcus viruses. Proc Natl Acad Sci USA 101:11013–11018
Lythgoe KA, Chao L (2003) Mechanisms of coexistence of a bacteria and a bacteriophage in a spatially homogeneous environment. Ecol Lett 6:326–334
McAdams HH, Arkin A (1997) Stochastic mechanisms in gene expression. Proc Natl Acad Sci USA 94:814–819
Middleboe M (2000) Bacterial growth rate and marine virus-host dynamics. Microb Ecol 40:114–124
Moebus K (1996) Marine bacteriophage reproductiong under nutrient-limited growth of host bacteria. I. Investigations with six phage-host systems. Mar Ecol Prog Ser 144:1–12
Murray JD (2002) Mathematical biology I. An introduction, 3rd edn. Springer, Berlin Heidelberg New York
Ricciuti CP (1972) Host-virus interactions in Escherichia coli: effect of stationary phase on viral release from MS2-infected bacteria. J Virol 10:162–165
Sano E, Carlson S, Wegley L, Rohwer F (2004) Movement of viruses between biomes. Appl Environ Microbiol 70: 5842–5846
Silander OK, Weinrich DM, Wright KM, O’Keefe KJ, Rang CU, Turner PE, Chao L (2005) Widespread genetic exchange among terrestrial bacteriophages. Proc Natl Acad Sci USA 102: 19009–19014
Sillankorva S, Oliveira R, Vieira MJ, Sutherland I, Azeredo J (2004) Pseudomonas flourescens infection by bacteriophage ΦS1: the influence of temperature, host growth phase and media. FEMS Microbiol Lett 241:13–20
Sullivan MB, Coleman M, Weigele P, Rohwer F, Chisholm SW (2005) Three Prochlorococcus cyanophage genomes: signature features and ecological interpretations. PLoS Biol 3:e144
Suttle CA (1994) The significance of viruses to mortality in aquatic microbial communities. Microb Ecol 28:237–243
Turner PE, Chao L (1999) Prisoner’s dilemma in an RNA virus. Nature 398:441–443
Wang I, Dykhuizen DE, Slobodkin LB (1996) The evolution of phage lysis timing. Evol Ecol 10:545–558
Weinbauer MG (2004) Ecology of prokaryotic viruses. FEMS Microbiol Rev 28:127–181
Weitz JS, Hartman H, Levin SA (2005) Coevolutionary arms races between bacteria and bacteriophage. Proc Natl Acad Sci USA 102:9535–9540
Acknowledgements
We are pleased to acknowledge the support of the Defense Advanced Research Projects Agency under grant HR0011-05-1-0057 to Princeton University. Joshua S. Weitz, Ph.D., holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. The authors would like to thank Simon Levin, Steve Pacala, and the members of the Theoretical Ecology Lab at Princeton University, where this work was initiated, for many inspiring discussions. The authors acknowledge A. Handel, A. Rabinovitch, F. Rohwer, P. Salamon, and two anonymous reviewers for helpful suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weitz, J.S., Dushoff, J. Alternative stable states in host–phage dynamics. Theor Ecol 1, 13–19 (2008). https://doi.org/10.1007/s12080-007-0001-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12080-007-0001-1