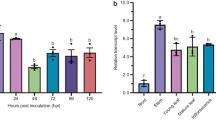
Abstract
A plant that is in part infected by a pathogen is more resistant throughout its whole body to subsequent infections – a phenomenon known as systemic acquired resistance (SAR). Mobile signals are synthesized at the site of infection and distributed throughout the plant through vascular tissues. Mechanism of SAR development subsequent to reaching the mobile signal in the distal tissue is largely unknown. Recently we showed that FLOWERING LOCUS D (FLD) gene of Arabidopsis thaliana is required in the distal tissue to activate SAR. FLD codes for a homologue of human-lysine-specific histone demethylase. Here we show that FLD function is required for priming (SAR induced elevated expression during challenge inoculation) of WRKY29 and WRKY6 genes. FLD also differentially influences basal and SAR-induced expression of WRKY38, WRKY65 and WRKY53 genes. In addition, we also show that FLD partly localizes in nucleus and influences histone modifications at the promoters of WRKY29 and WRKY6 genes. The results altogether indicate to the possibility of FLD’s involvement in epigenetic regulation of SAR.




Similar content being viewed by others
References
Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM and Sheen J 2002 MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415 977–983
Bhattacharjee S, Garner CM and Gassmann W 2013 New clues in the nucleus: transcriptional reprogramming in effector-triggered immunity. Front. Plant Sci. 4 364
Bonardi V, Tang S, Stallmann A, Roberts M, Cherkis K and Dangl JL 2011 Expanded functions for a family of plant intracellular immune receptors beyond specific recognition of pathogen effectors. Proc. Natl. Acad. Sci. USA 108 16463–16468
Cao H, Bowling SA, Gordon AS and Dong X 1994 Characterization of an Arabidopsis Mutant That Is Nonresponsive to Inducers of Systemic Acquired Resistance. Plant Cell 6 1583–1592
Chanda B, Xia Y, Mandal MK, Yu K, Sekine KT, Gao QM, Selote D, Hu Y, Stromberg A, Navarre D, Kachroo A and Kachroo P 2011 Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 43 421–427
Chaturvedi R, Krothapalli K, Makandar R, Nandi A, Sparks AA, Roth MR, Welti R and Shah J 2008 Plastid omega3-fatty acid desaturase-dependent accumulation of a systemic acquired resistance inducing activity in petiole exudates of Arabidopsis thaliana is independent of jasmonic acid. Plant J. 54 106–117
Chaturvedi R, Venables B, Petros RA, Nalam V, Li M, Wang X, Takemoto LJ and Shah J 2012 An abietane diterpenoid is a potent activator of systemic acquired resistance. Plant J. 71 161–172
Chen L, Song Y, Li S, Zhang L, Zou C and Yu D 2012 The role of WRKY transcription factors in plant abiotic stresses. Biochim. Biophys. Acta 1819 120–128
Conrath U 2011 Molecular aspects of defence priming. Trends Plant Sci. 16 524–531
Dempsey DA and Klessig DF 2012 SOS - too many signals for systemic acquired resistance? Trends Plant Sci. 17 538–545
Durrant WE and Dong X 2004 Systemic acquired resistance. Annu. Rev. Phytopathol. 42 185–209
Eulgem T and Somssich IE 2007 Networks of WRKY transcription factors in defense signaling. Curr. Opin. Plant Biol. 10 366–371
Fu ZQ and Dong X 2013 Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 64 839–863
Giri MK, Swain S, Gautam JK, Singh S, Singh N, Bhattacharya L and Nandi AK 2014 The Arabidopsis thaliana At4g13040 gene, a unique member of the AP2/EREBP family, is a positive regulator for salicylic acid accumulation and basal defense against bacterial pathogens. J Plant Physiol. doi:10.1016/j.jplph.2013.12.015
He Y, Michaels SD and Amasino RM 2003 Regulation of flowering time by histone acetylation in Arabidopsis. Science 302 1751–1754
Jaskiewicz M, Conrath U and Peterhansel C 2011 Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 12 50–55
Jung HW, Tschaplinski TJ, Wang L, Glazebrook J and Greenberg JT 2009 Priming in systemic plant immunity. Science 324 89–91
Kim KC, Fan B and Chen Z 2006 Pathogen-induced Arabidopsis WRKY7 is a transcriptional repressor and enhances plant susceptibility to Pseudomonas syringae. Plant Physiol. 142 1180–1192
Kim KC, Lai Z, Fan B and Chen Z 2008 Arabidopsis WRKY38 and WRKY62 transcription factors interact with histone deacetylase 19 in basal defense. Plant Cell 20 2357–2371
Liu F, Quesada V, Crevillen P, Baurle I, Swiezewski S and Dean C 2007 The Arabidopsis RNA-binding protein FCA requires a lysine-specific demethylase 1 homolog to downregulate FLC. Mol. Cell 28 398–407
Liu PP, von Dahl CC, Park SW and Klessig DF 2011 Interconnection between methyl salicylate and lipid-based long-distance signaling during the development of systemic acquired resistance in Arabidopsis and tobacco. Plant Physiol. 155 1762–1768
Luna E, Bruce TJ, Roberts MR, Flors V and Ton J 2012 Next-generation systemic acquired resistance. Plant Physiol. 158 844–853
Maldonado AM, Doerner P, Dixon RA, Lamb CJ and Cameron RK 2002 A putative lipid transfer protein involved in systemic resistance signalling in Arabidopsis. Nature 419 399–403
Manosalva PM, Park SW, Forouhar F, Tong L, Fry WE and Klessig DF 2010 Methyl esterase 1 (StMES1) is required for systemic acquired resistance in potato. Mol. Plant Microb. Interact. 23 1151–1163
Nandi A, Welti R and Shah J 2004 The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance. Plant Cell 16 465–477
Navarova H, Bernsdorff F, Doring AC and Zeier J 2012 Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24 5123–5141
Pandey SP and Somssich IE 2009 The role of WRKY transcription factors in plant immunity. Plant Physiol 150 1648–1655
Park SW, Kaimoyo E, Kumar D, Mosher S and Klessig DF 2007 Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318 113–116
Rasmussen JB, Hammerschmidt R and Zook MN 1991 Systemic induction of salicylic acid accumulation in cucumber after inoculation with Pseudomonas syringae pv syringae. Plant Physiol. 97 1342–1347
Robatzek S and Somssich IE 2002 Targets of AtWRKY6 regulation during plant senescence and pathogen defense. Genes Dev. 16 1139–1149
Ross AF 1961 Systemic acquired resistance induced by localized virus infections in plants. Virology 14 340–358
Saleh A, Alvarez-Venegas R and Avramova Z 2008 An efficient chromatin immunoprecipitation (ChIP) protocol for studying histone modifications in Arabidopsis plants. Nat. Protocols 3 1018–1025
Shah J 2009 Plants under attack: systemic signals in defence. Curr. Opin. Plant Biol. 12 459–464
Singh V, Roy S, Giri MK, Chaturvedi R, Chowdhury Z, Shah J and Nandi AK 2013 Arabidopsis thaliana FLOWERING LOCUS D is required for systemic acquired resistance. Mol. Plant Microb. Interact. 26 1079–1088
Slaughter A, Daniel X, Flors V, Luna E, Hohn B and Mauch-Mani B 2012 Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 158 835–843
Spoel SH and Dong X 2012 How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 12 89–100
Sticher L, Mauch-Mani B and Metraux JP 1997 Systemic acquired resistance. Annu. Rev. Phytopathol. 35 235–270
Swain S, Roy S, Shah J, Van Wees S, Pieterse CM and Nandi AK 2011 Arabidopsis thaliana cdd1 mutant uncouples the constitutive activation of salicylic acid signalling from growth defects. Mol. Plant Pathol. 12 855–865
Tsuchiya Y, Vidaurre D, Toh S, Hanada A, Nambara E, Kamiya Y, Yamaguchi S and McCourt P 2010 A small-molecule screen identifies new functions for the plant hormone strigolactone. Nat. Chem. Biol. 6 741–749
Vlot AC, Liu PP, Cameron RK, Park SW, Yang Y, Kumar D, Zhou F, Padukkavidana T, Gustafsson C, Pichersky E and Klessig DF 2008 Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J. 56 445–456
Wildermuth MC, Dewdney J, Wu G and Ausubel FM 2001 Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414 562–565
Xin XF and He SY 2013 Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 51 473–498
Xu X, Chen C, Fan B and Chen Z 2006 Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors. Plant Cell 18 1310–1326
Yu CW, Liu X, Luo M, Chen C, Lin X, Tian G, Lu Q, Cui Y and Wu K 2011 HISTONE DEACETYLASE6 interacts with FLOWERING LOCUS D and regulates flowering in Arabidopsis. Plant Physiol. 156 173–184
Acknowledgements
This work is supported by Department of Biotechnology (DBT) grant (BT/PR14656/BRB/10/864/2010) to AKN, University Grant Commission merit fellowship to DS and Council for Scientific and Industrial Research fellowship to SR. We acknowledge Jyoti Shah for comments and help in manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Corresponding editor: Utpal Nath
[Singh V, Roy S, Singh D and Nandi AK 2014 Arabidopsis FLOWERING LOCUS D influences systemic-acquired-resistance-induced expression and histone modifications of WRKY genes. J. Biosci. 39 1–8] DOI 10.1007/s12038-013-9407-7
Supplementary materials pertaining to this article are available on the Journal of Biosciences Website at http://www.ias.ac.in/jbiosci/mar2014/supp/Singh.pdf
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 38.2 kb)
Rights and permissions
About this article
Cite this article
Singh, V., Roy, S., Singh, D. et al. Arabidopsis FLOWERING LOCUS D influences systemic-acquired-resistance-induced expression and histone modifications of WRKY genes. J Biosci 39, 119–126 (2014). https://doi.org/10.1007/s12038-013-9407-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-013-9407-7