Abstract
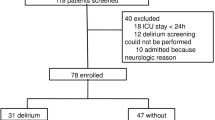
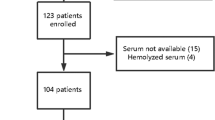
There are different theories about the pathophysiology of sepsis-associated encephalopathy (SAE), and the majority of our knowledge was derived from critically ill patients. 7In less severe sepsis, it is probable that neuroinflammation can be a major aspect of SAE development. We hypothesized that in non-severe septic patients, blood biomarkers of inflammation, endothelial activation, coagulation, and brain function would be different when compared to patients with and without brain dysfunction. A total of 30 patients presenting with community-acquired pneumonia (CAP)-induced sepsis were included of which 10 (33 %) developed SAE. Eight medical patients admitted to the general ward, except due to sepsis or infection, which developed delirium were included as delirium, non-sepsis group. From all measured biomarkers, only brain-derived neurotrophic factor (BDNF), regulated upon activation normal T cell expressed, and presumably secreted (RANTES), and interleukin (IL)-10 where significantly different when compared to SAE and sepsis groups. In addition, SAE patients presented higher levels of BDNF, vascular cellular adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), platelet-derived growth factor (PDGF)-AB/BB and RANTES when compared to delirium patients. In conclusion, the profile of biomarkers differs between SAE, sepsis, and delirium patients, suggesting that pathways related to SAE are different from delirium and from sepsis itself.
Similar content being viewed by others
References
Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, Chretien F, Sharshar T (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3:15
Shah FA, Pike F, Alvarez K, Angus D, Newman AB, Lopez O, Tate J, Kapur V et al (2013) Bidirectional relationship between cognitive function and pneumonia. Am J Respir Crit Care Med 188:586–92
Levy MM, Fink MP, Marshall JC, International Sepsis Definitions Conference (2001) SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference (2003). Intensive Care Med 29:530–538
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI (1990) Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 113:941–8
Cunninghamn C, Maclullich AM (2013) At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun 28:1–13
van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, Pickkers P (2011) Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective dysfunction: indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care 15:R297
Ritter C, Tomasi CD, Dal-Pizzol F, Pinto BB, Dyson A, de Miranda AS, Comim CM, Soares M et al (2014) Inflammation biomarkers and delirium in critically ill patients. Crit Care 18:R106
Berg RM, Plovsing RR, Ronit A, Bailey DM, Holstein-Rathlou NH, Moller K (2012) Disassociation of static and dynamic cerebral autoregulatory performance in healthy volunteers after LPS infusion and in patients with sepsis. Am J Physiol Regul Integr Comp Physiol 303:R1127–35
Hughes CG, Morandi A, Girard TD, Riedel B, Thompson JL, Shintani AK, Pun BT, Ely EW et al (2013) Association between endothelial dysfunction and acute brain dysfunction during critical illness. Anesthesiology 118:631–9
Rosengarten B, Krekel D, Kuhnert S, Schulz R (2012) Early neurovascular uncoupling in the brain during community acquired pneumonia. Crit Care 16:R64
Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, Huyghens L (2006) Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med 34:1967–74
Piazza O, Russo E, Cotena S, Esposito G, Tufano R (2007) Elevated S100B levels do not correlate with the severity of encephalopathy during sepsis. Br J Anaesth 99:518–21
Ritter C, Miranda AS, Giombelli VR, Tomasi CD, Comim CM, Teixeira AL, Quevedo J, Dal-Pizzol F (2012) Brain-derived neurotrophic factor plasma levels are associated with mortality in critically ill patients even in the absence of brain injury. Crit Care 16:R234
Grandi C, Tomasi CD, Fernandes K, Stertz L, Kapczinski F, Quevedo J, Dal-Pizzol F, Ritter C (2011) Brain-derived neurotrophic factor and neuron-specific enolase, but not S100β, levels are associated to the occurrence of delirium in intensive care unit patients. J Crit Care 26:133–7
Lim JY, Reighard CP, Crowther DC (2015) The pro-domains of neurotrophins, including BDNF, are linked to Alzheimer’s disease through a toxic synergy with A-beta. Hum Mol Genet 24:3929–3938
Acknowledgments
The Translational Psychiatry Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, The University of Texas Health Science Center at Houston (UTHealth) Medical School. Laboratory of Neurosciences and Laboratório de Fisiopatologia Experimental (Brazil) are part of the centers of the National Institute for Molecular Medicine (INCT-MM) and the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Institutional Review Board of São José Hospital approved the study, and all subjects gave written informed consent.
Role of Funding Source
None of the funding source has role in study design or any scientific decision about the conduction of the study.
Disclosure Statement
The authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Tomasi, C.D., Vuolo, F., Generoso, J. et al. Biomarkers of Delirium in a Low-Risk Community-Acquired Pneumonia-Induced Sepsis. Mol Neurobiol 54, 722–726 (2017). https://doi.org/10.1007/s12035-016-9708-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-9708-6