Abstract
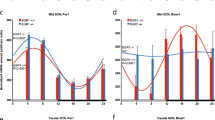
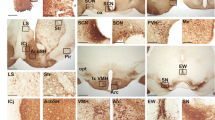
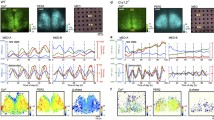
Circadian rhythms, generated in the mouse suprachiasmatic nucleus (SCN), are synchronized to the environmental day–night changes by photic input. The activation of the extracellular signal-regulated kinases 1 and 2 (ERK1,2) and cAMP response element-binding protein (CREB)-mediated transcription play a critical role in this photoentrainment. The small GTPase Ras is one of the major upstream regulators of the ERK1,2/CREB pathway. In contrast to the well-described role of Ras in structural and functional synaptic plasticity in the adult mouse brain, the physiological regulation of Ras by photic sensory input is yet unknown. Here, we describe for the first time a circadian rhythm of Ras activity in the mouse SCN. Using synRas transgenic mice, expressing constitutively activated V12-Ha-Ras selectively in neurons, we demonstrate that enhanced Ras activation causes shortening of the circadian period length. We found upregulated expression and decreased inhibitory phosphorylation of the circadian period length modulator, glycogen synthase kinase-3 beta (GSK3β), in the SCN of synRas mice. Conversely, downregulation of Ras activity by blocking its function with an antibody in oscillating cell cultures reduced protein levels and increased phosphorylation of GSK3β and lengthened the period of BMAL1 promoter-driven luciferase activity. Furthermore, enhanced Ras activity in synRas mice resulted in a potentiation of light-induced phase delays at early subjective night, and increased photic induction of pERK1,2/pCREB and c-Fos. In contrast, at late subjective night, photic activation of Ras/ERK1,2/CREB in synRas mice was not sufficient to stimulate c-Fos protein expression and phase advance the clock. Taken together, our results demonstrate that Ras activity fine tunes the period length and modulates photoentrainment of the circadian clock.







Similar content being viewed by others
References
Katz ME, McCormick F (1997) Signal transduction from multiple Ras effectors. Curr Opin Genes Dev 7(1):75–79
Marshall CJ (1996) Ras effectors. Curr Opin Cell Biol 8(2):197–204
Heumann R, Goemans C, Bartsch D, Lingenhohl K, Waldmeier PC, Hengerer B, Allegrini PR, Schellander K et al (2000) Transgenic activation of Ras in neurons promotes hypertrophy and protects from lesion-induced degeneration. J Cell Biol 151(7):1537–1548
Arendt T, Gartner U, Seeger G, Barmashenko G, Palm K, Mittmann T, Yan L, Hummeke M et al (2004) Neuronal activation of Ras regulates synaptic connectivity. Eur J Neurosci 19(11):2953–2966. doi:10.1111/j.0953-816X.2004.03409.x
Manns M, Bichler Z, Leske O, Heumann R (2010) Neuronal Ras activation inhibits adult hippocampal progenitor cell division and impairs spatial short-term memory. Genes Brain Behav 9(5):525–536. doi:10.1111/j.1601-183X.2010.00584.x
Golombek DA, Rosenstein RE (2010) Physiology of circadian entrainment. Physiol Rev 90(3):1063–1102. doi:10.1152/physrev.00009.2009
Albrecht U (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74(2):246–260. doi:10.1016/j.neuron.2012.04.006
Coogan AN, Piggins HD (2004) MAP kinases in the mammalian circadian system—key regulators of clock function. J Neurochem 90(4):769–775
Obrietan K, Impey S, Storm DR (1998) Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci 1(8):693–700
Serchov T, Heumann R (2006) Constitutive activation of ras in neurons: implications for the regulation of the mammalian circadian clock. Chronobiol Int 23(1–2):191–200
Marais R, Marshall CJ (1996) Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv 27:101–125
Guan KL (1994) The mitogen activated protein kinase signal transduction pathway: from the cell surface to the nucleus. Cell Signal 6(6):581–589
Coogan AN, Piggins HD (2003) Circadian and photic regulation of phosphorylation of ERK1/2 and Elk-1 in the suprachiasmatic nuclei of the Syrian hamster. J Neurosci 23(7):3085–3093
Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME (1993) Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science (New York NY) 260(5105):238–241
Obrietan K, Impey S, Smith D, Athos J, Storm DR (1999) Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem 274(25):17748–17756
Abe H, Rusak B (1994) Physiological mechanisms regulating photic induction of Fos-like protein in hamster suprachiasmatic nucleus. Neurosci Biobehav Rev 18(4):531–536
Dziema H, Oatis B, Butcher GQ, Yates R, Hoyt KR, Obrietan K (2003) The ERK/MAP kinase pathway couples light to immediate-early gene expression in the suprachiasmatic nucleus. Eur J Neurosci 17(8):1617–1627
Rusak B, McNaughton L, Robertson HA, Hunt SP (1992) Circadian variation in photic regulation of immediate-early gene mRNAs in rat suprachiasmatic nucleus cells. Brain Res Mol Brain Res 14(1–2):124–130
Rusak B, Robertson HA, Wisden W, Hunt SP (1990) Light pulses that shift rhythms induce gene expression in the suprachiasmatic nucleus. Science (New York NY) 248(4960):1237–1240
Albrecht U, Sun ZS, Eichele G, Lee CC (1997) A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 91(7):1055–1064
Tischkau SA, Gallman EA, Buchanan GF, Gillette MU (2000) Differential cAMP gating of glutamatergic signaling regulates long-term state changes in the suprachiasmatic circadian clock. J Neurosci 20(20):7830–7837
Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P (2002) Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci U S A 99(11):7728–7733
Zylka MJ, Shearman LP, Weaver DR, Reppert SM (1998) Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron 20(6):1103–1110
Butcher GQ, Doner J, Dziema H, Collamore M, Burgoon PW, Obrietan K (2002) The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J Biol Chem 277(33):29519–29525
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418(6901):935–941
Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, Young MW (1998) The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell 94(1):97–107
Iitaka C, Miyazaki K, Akaike T, Ishida N (2005) A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem 280(33):29397–29402
Sahar S, Zocchi L, Kinoshita C, Borrelli E, Sassone-Corsi P (2010) Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation. PLoS One 5(1):e8561. doi:10.1371/journal.pone.0008561
Harada Y, Sakai M, Kurabayashi N, Hirota T, Fukada Y (2005) Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J Biol Chem 280(36):31714–31721
Hirota T, Lewis WG, Liu AC, Lee JW, Schultz PG, Kay SA (2008) A chemical biology approach reveals period shortening of the mammalian circadian clock by specific inhibition of GSK-3beta. Proc Natl Acad Sci U S A 105(52):20746–20751
Zhang JS, Koenig A, Harrison A, Ugolkov AV, Fernandez-Zapico ME, Couch FJ, Billadeau DD (2011) Mutant K-Ras increases GSK-3beta gene expression via an ETS-p300 transcriptional complex in pancreatic cancer. Oncogene 30(34):3705–3715. doi:10.1038/onc.2011.90
Holzer M, Gartner U, Klinz FJ, Narz F, Heumann R, Arendt T (2001) Activation of mitogen-activated protein kinase cascade and phosphorylation of cytoskeletal proteins after neurone-specific activation of p21ras. I Mitogen-activated protein kinase cascade. Neuroscience 105(4):1031–1040
Capon DJ, Chen EY, Levinson AD, Seeburg PH, Goeddel DV (1983) Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature 302(5903):33–37
Sauerwald A, Hoesche C, Oschwald R, Kilimann MW (1990) The 5′-flanking region of the synapsin I gene. A G + C-rich, TATA- and CAAT-less, phylogenetically conserved sequence with cell type-specific promoter function. J Biol Chem 265(25):14932–14937
Werge TM, Biocca S, Cattaneo A (1990) Intracellular immunization. Cloning and intracellular expression of a monoclonal antibody to the p21ras protein. FEBS Lett 274(1–2):193–198
Takahashi K, Sawasaki Y, Hata J, Mukai K, Goto T (1990) Spontaneous transformation and immortalization of human endothelial cells. Vitro Cell Dev Biol 26(3 Pt 1):265–274
Jilg A, Lesny S, Peruzki N, Schwegler H, Selbach O, Dehghani F, Stehle JH (2010) Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus 20(3):377–388. doi:10.1002/hipo.20637
Butcher GQ, Lee B, Obrietan K (2003) Temporal regulation of light-induced extracellular signal-regulated kinase activation in the suprachiasmatic nucleus. J Neurophysiol 90(6):3854–3863
Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN (2003) The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat Neurosci 6(2):111–112. doi:10.1038/nn1006
Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS (1990) Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron 5(2):127–134
Harwood AJ (2001) Regulation of GSK-3: a cellular multiprocessor. Cell 105(7):821–824
Balsalobre A, Damiola F, Schibler U (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93(6):929–937
Akashi M, Nishida E (2000) Involvement of the MAP kinase cascade in resetting of the mammalian circadian clock. Genes Dev 14(6):645–649
Gartner U, Alpar A, Behrbohm J, Heumann R, Arendt T (2005) Enhanced Ras activity promotes spine formation in synRas mice neocortex. Neuroreport 16(2):149–152
Gartner U, Alpar A, Reimann F, Seeger G, Heumann R, Arendt T (2004) Constitutive Ras activity induces hippocampal hypertrophy and remodeling of pyramidal neurons in synRas mice. J Neurosci Res 77(5):630–641. doi:10.1002/jnr.20194
Seeger G, Gartner U, Arendt T (2005) Transgenic activation of Ras in neurons increases synapse formation in mouse neocortex. J Neural Transm 112(6):751–761. doi:10.1007/s00702-004-0226-8
Lafenetre P, Leske O, Ma-Hogemeie Z, Haghikia A, Bichler Z, Wahle P, Heumann R (2010) Exercise can rescue recognition memory impairment in a model with reduced adult hippocampal neurogenesis. Front Behav Neurosci 3:34. doi:10.3389/neuro.08.034.2009
Curtis J, Finkbeiner S (1999) Sending signals from the synapse to the nucleus: possible roles for CaMK, Ras/ERK, and SAPK pathways in the regulation of synaptic plasticity and neuronal growth. J Neurosci Res 58(1):88–95
Waltereit R, Weller M (2003) Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol 27(1):99–106. doi:10.1385/MN:27:1:99
Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU (1994) Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science (New York NY) 266(5191):1713–1717
Farnsworth CL, Freshney NW, Rosen LB, Ghosh A, Greenberg ME, Feig LA (1995) Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature 376(6540):524–527
Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, Yang L, Mao L (2004) Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol Neurobiol 29(1):1–14
Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB (1998) A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 20(5):895–904
Oh JS, Manzerra P, Kennedy MB (2004) Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J Biol Chem 279(17):17980–17988
Shimizu K, Okada M, Nagai K, Fukada Y (2003) Suprachiasmatic nucleus circadian oscillatory protein, a novel binding partner of K-Ras in the membrane rafts, negatively regulates MAPK pathway. J Biol Chem 278(17):14920–14925
Heumann R (1994) Neurotrophin signalling. Curr Opin Neurobiol 4(5):668–679
Liang FQ, Allen G, Earnest D (2000) Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J Neurosci 20(8):2978–2987
Liang FQ, Walline R, Earnest DJ (1998) Circadian rhythm of brain-derived neurotrophic factor in the rat suprachiasmatic nucleus. Neurosci Lett 242(2):89–92
Price JL, Blau J, Rothenfluh A, Abodeely M, Kloss B, Young MW (1998) Double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94(1):83–95
Lavoie J, Hebert M, Beaulieu JM (2013) Glycogen synthase kinase-3beta haploinsufficiency lengthens the circadian locomotor activity period in mice. Behav Brain Res 253:262–265. doi:10.1016/j.bbr.2013.08.001
Paul JR, Johnson RL, Jope RS, Gamble KL (2012) Disruption of circadian rhythmicity and suprachiasmatic action potential frequency in a mouse model with constitutive activation of glycogen synthase kinase 3. Neuroscience 226:1–9. doi:10.1016/j.neuroscience.2012.08.047
Akashi M, Hayasaka N, Yamazaki S, Node K (2008) Mitogen-activated protein kinase is a functional component of the autonomous circadian system in the suprachiasmatic nucleus. J Neurosci 28(18):4619–4623. doi:10.1523/JNEUROSCI. 3410-07.2008
Cao R, Butcher GQ, Karelina K, Arthur JS, Obrietan K (2013) Mitogen- and stress-activated protein kinase 1 modulates photic entrainment of the suprachiasmatic circadian clock. Eur J Neurosci 37(1):130–140. doi:10.1111/ejn.12028
Butcher GQ, Lee B, Hsieh F, Obrietan K (2004) Light- and clock-dependent regulation of ribosomal S6 kinase activity in the suprachiasmatic nucleus. Eur J Neurosci 19(4):907–915
Acknowledgments
This work was supported by the International Graduate School of Neuroscience of the Ruhr-University, Bochum, Germany. The authors acknowledge Soner Cinar and Beatrix Feldhaus (BSc and Diplom students from the Dept. of Molecular Neurobiochemistry, RUB, Germany) for their help with T-24 cell culture experiments and anti-Ras-scFv cloning, respectively. We thank the RUBION service center for providing facilities.
Conflict of Interest
The authors declare no competing financial interests.
Compliance with Ethical Standards
The authors declare that all animal procedures were performed in accordance to the German Law on Animal Welfare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tsvetan Serchov and Antje Jilg contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
Circadian regulation of MKP-1 protein expression in the SCN of WT and synRas mice. Representative Western blot and densitometric quantification of MKP-1 levels normalized to tubulin. Data were presented as a mean ±SEM (n=4; #p<0.05 in comparison to CT18 for both genotypes, two-way ANOVA with Bonferroni post hoc test). (GIF 44 kb)
Suppl. Fig. 2
Photic regulation of pERK1,2 and pCREB in the SCN of WT and synRas mice at early and late subjective night. a Representative Western blot and densitometric quantification of pERK1,2 normalized to the total ERK1,2. Representative immunohistochemical images of pCREB and densitometric quantification of pCREB induction in the SCN of WT and synRas animals at early subjective night (CT14) (b) and late subjective night (CT22) (c). Data are presented as mean ±SEM (n=4 per group, *p<0.05, **p<0.01 two-way ANOVA with Bonferroni post hoc test). (GIF 190 kb)
Suppl. Fig. 3
Representative Western blot demonstrating in vitro expression of anti-Ras-scFv antibody in T24 cells. (GIF 16 kb)
Rights and permissions
About this article
Cite this article
Serchov, T., Jilg, A., Wolf, C.T. et al. Ras Activity Oscillates in the Mouse Suprachiasmatic Nucleus and Modulates Circadian Clock Dynamics. Mol Neurobiol 53, 1843–1855 (2016). https://doi.org/10.1007/s12035-015-9135-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9135-0