Abstract
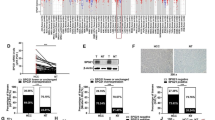
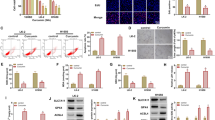
It was recently demonstrated that interleukin-6 (IL-6) induces the epithelial-to-mesenchymal transition (EMT) in cholangiocarcinoma (CCA), but the underlying molecular mechanism remains to be explored. In this study, we studied the role of suppresser of cytokine signaling 3 (SOCS3), a negative feedback regulator of IL-6/STAT3, in the IL-6-induced EMT in CCA. Treatment with IL-6 induced the EMT by decreasing the E-cadherin expression and increasing the expression of N-cadherin and vimentin. Using wound healing and invasion assays, we found that IL-6 promoted cell motility. Further, a stably transfected cell line overexpressing SOCS3 was constructed. Enhanced SOCS3 expression decreased IL-6-induced cell invasion and EMT in parallel with downregulating the IL-6/STAT3 pathway. In contrast, SOCS3 silencing using siRNA exhibited no effect on the cell invasive ability and EMT. Finally, an in vivo study indicated that the enhancement of SOCS3 expression decreased metastasis compared with the control, and this effect was achieved by the repression of p-STAT3, N-cadherin and vimentin, and the induction of E-cadherin assessed by Western blot analysis. Our results suggest that enhanced expression of SOCS3 can antagonize IL-6-induced EMT and cell metastasis by abrogating the IL-6/STAT3 pathway. These data establish that SOCS3 plays a role in the EMT in CCA and may provide novel therapeutic strategies for CCA.





Similar content being viewed by others
References
Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–29. doi:10.1053/j.gastro.2013.10.013.
DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–62. doi:10.1097/01.sla.0000251366.62632.d3.
Lau SH, Lau WY. Current therapy of hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2012;11(1):12–7. doi:10.1016/S1499-3872(11)60119-7.
Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi:10.1172/JCI39104.
Gores GJ. Cholangiocarcinoma: current concepts and insights. Hepatology. 2003;37(5):961–9. doi:10.1053/jhep.2003.50200.
Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology. 1994;20(2):376–82. doi:10.1002/hep.1840200217.
Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of Interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006;44(6):1055–65. doi:10.1016/j.jhep.2005.10.030.
Yokomuro S, Tsuji H, Lunz JG III, Sakamoto T, Ezure T, Murase N, et al. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin A: comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology. 2000;32(1):26–35. doi:10.1053/jhep.2000.8535.
Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24(2):139–54. doi:10.1055/s-2004-828891.
Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi:10.1016/j.ccr.2009.01.009.
Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, GuCCAiardi ME, Frank DA, et al. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42:1329–38. doi:10.1002/hep.20966.
Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940–7. doi:10.1038/onc.2009.180.
Yadav A, Kumar B, Datta J, Teknos TN, Kumar P. IL-6 promotes head and neck tumor metastasis by inducing epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol Cancer Res. 2011;9(12):1658–67. doi:10.1158/1541-7786.MCR-11-0271.
Zhao Z, Cheng X, Wang Y, Han R, Li L, Xiang T, et al. Metformin inhibits the IL-6-induced epithelial-mesenchymal transition and lung adenocarcinoma growth and metastasis. PLoS ONE. 2014;9(4):e95884. doi:10.1371/journal.pone.0095884.
Inagaki-Ohara K, Kondo T, Ito M, Yoshimura A. SOCS, inflammation, and cancer. JAK-STAT. 2013;2(3):e24053. doi:10.4161/jkst.24053.
Croker BA, Kiu H, Nicholson SE. SOCS regulation of the JAK/STAT signaling pathway. Semin Cell Dev Biol. 2008;19(4):414–22. doi:10.1016/j.semcdb.2008.07.010.
Weber A, Hengge UR, Bardenheuer W, Tischoff I, Sommerer F, Markwarth A, et al. SOCS-3 is frequently methylated in head and neck squamous cell carcinoma and its precursor lesions and causes growth inhibition. Oncogene. 2005;24:6699–708. doi:10.1038/sj.onc.1208818.
He B, You L, Uematsu K, Zang K, Xu Z, Lee AY, et al. SOCS-3 is frequently silenced by hypermethylation and suppresses cell growth in human lung cancer. Proc Natl Acad Sci USA. 2003;100(24):14133–8. doi:10.1073/pnas.2232790100.
Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24(42):6406–17. doi:10.1038/sj.onc.1208788.
Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, et al. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132(1):384–96. doi:10.1053/j.gastro.2006.10.037.
Rossa C Jr, Sommer G, Spolidorio LC, Rosenzweig SA, Watson DK, Kirkwood KL. Loss of expression and function of socs3 is an early event in HNSCC: altered subcellular localization as a possible mechanism involved in proliferation, migration and invasion. PLoS ONE. 2012;7(9):e45197. doi:10.1371/journal.pone.0045197.
Jiang GX, Zhong XY, Cui YF, Liu W, Tai S, Wang ZD, et al. IL-6/STAT3/TFF3 signaling regulates human biliary epithelial cell migration and wound healing in vitro. Mol Biol Rep. 2010;37(8):3813–8. doi:10.1007/s11033-010-0036-z.
Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6–7):303–14. doi:10.1002/emmm.200900043.
Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;1:i104–8. doi:10.1136/ard.2010.140145.
Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial–mesenchymal transition in ovarian carcinomas. Br J Cancer. 2009;100(1):134–44. doi:10.1038/sj.bjc.6604794.
Yamada D, Kobayashi S, Wada H, Kawamoto K, Marubashi S, Eguchi H, et al. Role of crosstalk between interleukin-6 and transforming growth factor-beta 1 in epithelial–mesenchymal transition and chemoresistance in biliary tract cancer. Eur J Cancer. 2013;49(7):1725–40. doi:10.1016/j.ejca.2012.12.002.
Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, et al. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes Cells. 1999;4(6):339–51. doi:10.1046/j.1365-2443.1999.00263.x.
Evans MK, Yu CR, Lohani A, Mahdi RM, Liu X, Trzeciak AR, et al. Expression of SOCS1 and SOCS3 genes is differentially regulated in breast cancer cells in response to proinflammatory cytokine and growth factor signals. Oncogene. 2007;26(13):1941–8. doi:10.1038/sj.onc.1209993.
Bellezza I, Neuwirt H, Nemes C, Cavarretta IT, Puhr M, Steiner H, et al. Suppressor of cytokine signaling-3 antagonizes cAMP effects on proliferation and apoptosis and is expressed in human prostate cancer. Am J Pathol. 2006;169(6):2199–208. doi:10.2353/ajpath.2006.060171.
Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi:10.1016/S1470-2045(14)70263-3.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81170426).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the study were conducted. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, QX., Jiang, XM., Wang, ZD. et al. Enhanced expression of suppresser of cytokine signaling 3 inhibits the IL-6-induced epithelial-to-mesenchymal transition and cholangiocarcinoma cell metastasis. Med Oncol 32, 105 (2015). https://doi.org/10.1007/s12032-015-0553-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-015-0553-7