Abstract
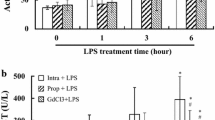
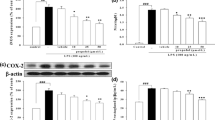
This study is set to explore the role of commonly used intravenous anesthetic propofol on the inflammatory response of rat liver Kupffer cells (KCs) induced by lipopolysaccharides (LPS). The isolated KCs were cultured at the density of 1 × 105/ml, divided into five groups randomly after 48 h culture: group C, control group; group L, KCs were treated with 1 μg/ml LPS for 24 h; groups P1, P2, P3, KCs were pretreated with propofol at low (25 μM), medium (50 μM), high (100 μM) concentration for 2 h, respectively, and then were stimulated with 1 μg/ml LPS for 24 h. The expressions of tumor necrosis factor-α (TNF-α) mRNA and interleukin-1β (IL-1β) mRNA of every group were measured by RT-PCR. Nuclear NF-ΚB p65 was determined by Western blot. The concentrations of IL-1β and TNF-α in supernatant were measured by ELISA. Compared with the group C, TNF-α mRNA and IL-1β mRNA in group L were significantly up-regulated and NF-ΚB p65 was significantly up-regulated after LPS treatment (P < 0.05). Meanwhile, TNF-α and IL-1β were also significantly increased (P < 0.05). With propofol the mRNA expressions of aforementioned inflammatory mediators were significantly down-regulated and NF-ΚB p65 was significantly inhibited in group P2 and P3 (P < 0.05), compared with group L. However, low propofol concentration did not exhibit any effect (group P1, P > 0.05). Propofol at medium and high concentration can counteract the LPS-induced inflammatory response in KCs by regulating NF-ΚB p65 protein expression.



Similar content being viewed by others
References
Yamamoto, T., Kaizu, C., Kawasaki, T., Hasegawa, G., Umezu, H., Ohashi, R., et al. (2008). Macrophage colony-stimulating factor is indispensable for repopulation and differentiation of Kupffer cells but not for splenic red pulp macrophages in osteopetrotic (op/op) mice after macrophage depletion. Cell and Tissue Research, 332(2), 245–256.
Ma, W., Wang, Z. R., Shi, L., & Yuan, Y. (2006). Expression of macrophage inflammatory protein-1alpha in Kupffer cells following liver ischemia or reperfusion injury in rats. World Journal of Gastroenterology, 12(24), 3854–3858.
Zhang, W. F., Wu, C. X., & Gong, J. P. (2013). Effect of kupffer cells on mechanisms of nonalcoholic fatty liver disease. Progress in Physiology, 44(5), 363–366.
Zigmond, E., Samia-Grinberg, S., Pasmanik-Chor, M., Brazowski, E., Shibolet, O., Halpern, Z., et al. (2014). Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. Journal of Immunology, 193(1), 344–353.
Hong, I. H., Han, S. Y., Ki, M. R., Moon, Y. M., Park, J. K., You, S. Y., et al. (2013). Inhibition of kupffer cell activity improves transplantation of human adipose-derived stem cells and liver functions. Cell Transplantation, 22(3), 447–459.
Feng, M., Wang, Q., Zhang, F., & Lu, L. (2012). Ex vivo induced regulatory T cells regulate inflammatory response of Kupffer cells by TGF-beta and attenuate liver ischemia reperfusion injury. International Immunopharmacology, 12(1), 189–196.
Aziz-Seible, R. S., Lee, S. M., Kharbanda, K. K., McVicker, B. L., & Casey, C. A. (2011). Ethanol feeding potentiates the pro-inflammatory response of Kupffer cells to cellular fibronectin. Alcoholism, Clinical and Experimental Research, 35(4), 717–725.
Budick-Harmelin, N., Dudas, J., Demuth, J., Madar, Z., Ramadori, G., & Tirosh, O. (2008). Triglycerides potentiate the inflammatory response in rat Kupffer cells. Antioxidants & Redox Signaling, 10(12), 2009–2022.
Engelmann, C., Wallenborn, J., Olthoff, D., Kaisers, U. X., & Ruffert, H. (2014). Propofol versus flunitrazepam for inducing and maintaining sleep in postoperative ICU patients. Indian Journal of Critical Care Medicine, 18(4), 212–219.
Furmaga, H., Park, H. J., Cooperrider, J., Baker, K. B., Johnson, M., Gale, J. T., et al. (2014). Effects of ketamine and propofol on motor evoked potentials elicited by intracranial microstimulation during deep brain stimulation. Frontiers in Systems Neuroscience, 8, 89.
Jin, Y., Zhao, X., Li, H., Wang, Z., & Wang, D. (2013). Effects of sevoflurane and propofol on the inflammatory response and pulmonary function of perioperative patients with one-lung ventilation. Experimental and Therapeutic Medicine, 6(3), 781–785.
Sugasawa, Y., Yamaguchi, K., Kumakura, S., Murakami, T., Suzuki, K., Nagaoka, I., et al. (2012). Effects of sevoflurane and propofol on pulmonary inflammatory responses during lung resection. Journal of Anesthesia, 26(1), 62–69.
Sung, E. G., Jee, D., Song, I. H., Kim, H. S., Bae, J. H., & Park, S. H. (2005). Propofol attenuates Kupffer cell activation during hypoxia-reoxygenation. Canadian Journal of Anaesthesia, 52(9), 921–926.
Gangopadhyay, A., Lazure, D. A., Kelly, T. M., & Thomas, P. (1996). Purification and analysis of an 80-kDa carcinoembryonic antigen-binding protein from Kupffer cells. Archives of Biochemistry and Biophysics, 328(1), 151–157.
Ogiku, M., Kono, H., Hara, M., Tsuchiya, M., & Fujii, H. (2011). Glycyrrhizin prevents liver injury by inhibition of high-mobility group box 1 production by Kupffer cells after ischemia-reperfusion in rats. The Journal of Pharmacology and Experimental Therapeutics, 339(1), 93–98.
Tapia, G., Santibanez, C., Farias, J., Fuenzalida, G., Varela, P., Videla, L. A., et al. (2010). Kupffer-cell activity is essential for thyroid hormone rat liver preconditioning. Molecular and Cellular Endocrinology, 323(2), 292–297.
Holt, M. P., Yin, H., & Ju, C. (2010). Exacerbation of acetaminophen-induced disturbances of liver sinusoidal endothelial cells in the absence of Kupffer cells in mice. Toxicology Letters, 194(1–2), 34–41.
Lau, D.T., Negash, A., Chen, J., Crochet, N., Sinha, M., Zhang, Y., et al. (2013). Innate immune tolerance and the role of kupffer cells in differential responses to interferon therapy among patients with HCV genotype 1 infection. Gastroenterology, 144(2), 402–13e12.
Jung, K., Kang, M., Park, C., Hyun Choi, Y., Jeon, Y., Park, S. H., et al. (2012). Protective role of V-set and immunoglobulin domain-containing 4 expressed on kupffer cells during immune-mediated liver injury by inducing tolerance of liver T- and natural killer T-cells. Hepatology, 56(5), 1838–1848.
Xu, L., Yin, W., Sun, R., Wei, H., & Tian, Z. (2014). Kupffer cell-derived IL-10 plays a key role in maintaining humoral immune tolerance in hepatitis B virus-persistent mice. Hepatology, 59(2), 443–452.
Marra, F. (2009). Selective inhibition of NF-kappaB in Kupffer cells: good, but not for everything. Gut, 58(12), 1581–1582.
Hoffmann, F., Sass, G., Zillies, J., Zahler, S., Tiegs, G., Hartkorn, A., et al. (2009). A novel technique for selective NF-kappaB inhibition in Kupffer cells: contrary effects in fulminant hepatitis and ischaemia-reperfusion. Gut, 58(12), 1670–1678.
Kojima, S., Negishi, Y., Tsukimoto, M., Takenouchi, T., Kitani, H., & Takeda, K. (2014). Purinergic signaling via P2X7 receptor mediates IL-1beta production in Kupffer cells exposed to silica nanoparticle. Toxicology, 321, 13–20.
Chen, X. L., Xia, Z. F., Wei, D., Ben, D. F., & Wang, Y. J. (2005). Role of p38MAPK signal transduction pathway in Kupffer cells production of TNF-alpha and IL-1beta in severely burned rats. Chinese Journal of Surgery, 43(3), 185–188.
Tasdogan, M., Memis, D., Sut, N., & Yuksel, M. (2009). Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. Journal of Clinical Anesthesia, 21(6), 394–400.
Kalimeris, K., Christodoulaki, K., Karakitsos, P., Batistatou, A., Lekka, M., Bai, M., et al. (2011). Influence of propofol and volatile anaesthetics on the inflammatory response in the ventilated lung. Acta Anaesthesiologica Scandinavica, 55(6), 740–748.
Tarassishin, L., Suh, H. S., & Lee, S. C. (2014). LPS and IL-1 differentially activate mouse and human astrocytes: Role of CD14. Glia., 62(6), 999–1013.
Lee, S. Y., Kim, H. J., & Han, J. S. (2013). Anti-inflammatory Effect of Oyster Shell Extract in LPS-stimulated Raw 264.7 Cells. Preventive nutrition and food science., 18(1), 23–29.
Yang, W. S., Jeong, D., Nam, G., Yi, Y. S., Yoon, D. H., Kim, T. W., et al. (2013). AP-1 pathway-targeted inhibition of inflammatory responses in LPS-treated macrophages and EtOH/HCl-treated stomach by Archidendron clypearia methanol extract. Journal of Ethnopharmacology, 146(2), 637–644.
Abshagen, K., Eipel, C., Kalff, J. C., Menger, M. D., & Vollmar, B. (2007). Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. American Journal of Physiology. Gastrointestinal and Liver Physiology, 292(6), G1570–G1577.
Peng, Y., Gallagher, S. F., Landmann, R., Haines, K., & Murr, M. M. (2006). The role of p65 NF-kappaB/RelA in pancreatitis-induced Kupffer cell apoptosis. Journal of Gastrointestinal Surgery, 10(6), 837–847.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, S., Wang, Cx., Liu, Nz. et al. Anti-inflammatory Effects of Propofol on Lipopolysaccharides-Treated Rat Hepatic Kupffer Cells. Cell Biochem Biophys 71, 845–850 (2015). https://doi.org/10.1007/s12013-014-0272-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0272-2