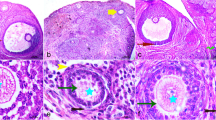
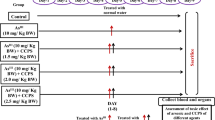
Abstract
Continuation of prolonged treatment against arsenicosis with conventional chelating therapy is a global challenge. The present study was intended to evaluate the defensive effect of arjunolic acid against arsenic-induced oxidative stress and female reproductive dysfunction. Wistar strain adult female rats were given sodium arsenite (10 mg/kg body weight) in combination with arjunolic acid (10 mg/kg body weight) orally for two estrous cycles. Electrozymographic analysis explored that arjunolic acid co-treatment counteracted As3+-induced ROS production in uterine tissue by stimulating the activities of endogenous enzymatic antioxidants. Arjunolic acid was able to enhance the protection against mutagenic uterine DNA breakage, necrosis, and ovarian–uterine tissue damages in arsenicated rats by improving the ovarian steroidogenesis. The mechanisms might be coupled with the augmentation of antioxidant defense system, partly through the elimination of arsenic with the involvement of S-adenosyl methionine pool where circulating levels of vitamin B12, folic acid, and homocysteine play critical roles as evidenced from our present investigation.




Similar content being viewed by others
References
Singh N, Kumar D, Sahu AP (2007) Arsenic in the environment: effects on human health and possible prevention. J Environ Biol 28:359–365
Maiti S, Chattopadhyay S, Deb B, Samanta T, Maji G, Pan B et al (2012) Antioxidant and metabolic impairment result in DNA damage in arsenic-exposed individuals with severe dermatological manifestations in Eastern India. Environ Toxicol 27:342–350
Roy P, Saha A (2002) Metabolism and toxicity of arsenic: a human carcinogen. Curr Sci 82:38–45
Rahman MM, Ng JC, Naidu R (2009) Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ Geochem Health 31:189–200
Tokar EJ, Tallaa LB, Ward JM, Lunn R, Sams RL, Waalkes MP (2010) Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit Rev Toxicol 40(10):912–927
Pott WA, Benjamin SA, Yang RS (2001) Pharmacokinetics, metabolism, and carcinogenicity of arsenic. Rev Environ Contam Toxicol 169:165–214
Sarkar M, Chaudhuri GR, Chattopadhyay A, Biswas NM (2003) Effect of sodium arsenite on spermatogenesis, plasma gonadotrophins and testosterone in rats. Asian J Androl 5:27–31
Mukherjee S, Mukhopadhyay P (2009) Studies on arsenic toxicity in male rat gonads and its protection by high dietary protein supplementation. Al Ameen J Med Sci 2:73–77
Sanghamitra S, Hazra J, Upadhyay SN, Singh RK, Amal RC (2008) Arsenic induced toxicity on testicular tissue of mice. Indian J of Physio And Pharm 52:84–90
Chinoy N, Tewari K, Jhala D (2004) Fluoride and/or arsenic toxicity in mice testis with formation of giant cells and subsequent recovery by some antidotes. Fluoride 37:172–184
Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A et al (2001) Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect 109:629–631
Zadorozhnaja TD, Little RE, Miller RK, Mendel NA, Taylor RJ, Presley BJ et al (2000) Concentrations of arsenic, cadmium, copper, lead, mercury, and zinc in human placentas from two cities in Ukraine. J Toxicol Environ Health A 61:255–263
Sen J, Chaudhuri AB (2008) Arsenic exposure through drinking water and its effect on pregnancy outcome in Bengali women. Arh Hig Rada Toksikol 599:271–275
Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AM et al (2009) Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicol Lett 185:197–202
Rodriguez KF, Ungewitter EK, Crespo-Mejias Y, Liu C, Nicol B, Kissling GE et al (2016) Effects of in utero exposure to arsenic during the second half of gestation on reproductive end points and metabolic parameters in female CD-1 mice. Environ Health Perspect 124:336–343
Chattopadhyay S, Ghosh S, Chaki S, Debnath J, Ghosh D (1999) Effect of sodium arsenite on plasma levels of gonadotrophins and ovarian steroidogenesis in mature albino rats, duration dependent response. J Toxicol Sci 24:425–431
Ghosh D, Chattopadhyay S, Debnath J (1999) Effect of sodium arsenite on adrenocortical activity in immature female rats, evidence of dose dependent response. J Environ Sci 11:419–422
Luetscher JA, Eagle H, Longcope WT, Watson EB (1946) Clinical uses of 2, 3-dimercaptopropanol (BAL). VIII. The effect of BAL on the excretion of arsenic in arsenical intoxication. J Clin Investig 25:534–540
Carleton AB, Peters RA, Thompson RHS (1948) The treatment of arsenical dermatitis with dimercaptopropanol (BAL). Q J Med 17:49–79
Bhattacharjee S, Pal S (2014) Additive protective effects of selenium and vitamin E against arsenic induced lipidemic and cardiotoxic effects in mice. Int J Pharm Pharm Sci 6:406–413
Islam MS, Awal MA, Mostofa M, Begum F, Khair A, Myenuddin M (2009) Effect of spirulina on toxic signs, body weight and hematological parameters in arsenic induced toxicities in ducks. Int J Poult Sci 8:75–79
Momotaj H, Hussain AZMI (2001) Effect of spirulina on arsenicosis patients in Bangladesh. Presentation prepared for Arsenic in Drinking Water: an International Conference at Columbia University, New York, pp 26–27
Sayed MA, Gofur MR, Khair A, Awal MA (2015) Protective role of spirulina and vitamin E against arsenic toxicity in rats. Asian Journal of Animal Sciences 9:330–340
Chattopadhyay S, Maiti S, Maji G, Deb B, Pan B, Ghosh D (2010) Protective role of Moringa oleifera (Sajina) seed on arsenic-induced hepatocellular degeneration in female albino rats. Biol Trace Elem Res 142:200–212
Mona A, Damegh A, Zeitoun MM, Salam AMA (2014) The role of fermented milk containing probiotic, dandelion as prebiotic or their combination on serum metabolites, enzymes, testosterone and testicular histopathology of arsenic-intoxicated male rats. Journal of Basic & Applied Sciences 10:492–503
Brahmachari G (2015) Beneficial effect of naturally occurring antioxidants against oxidative stress. In: Brahmachari G (ed) Bioactive natural products: chemistry and biology. Wiley, Delhi, pp 210–211
Hemalatha T, Pulavendran S, Balachandran C, Manohar BM, Puvanakrishnan R (2010) Arjunolic acid: a novel phytomedicine with multifunctional therapeutic applications. Indian J Exp Biol 48:238–247
Khan N, Haque M, Haque IU, Ali S, Malik MZ, Siddiqui MS et al (2015) Effect of Terminalia arjuna against arsenic-induced renal toxicity in mice. BAOJ Biotech 1:005
Vasanthi P, Parameswari CS (2012) Arjunolic acid attenuates cyclosporine induced nephrotoxicity-mitochondrial study. J Pharm Res 5:3729–3733
Sinha M, Manna P, Sil PC (2008) Protective effect of arjunolic acid against arsenic-induced oxidative stress in mouse brain. J Biochem Mol Toxicol 22:15–26
Manna P, Sinha M, Sil PC (2007) Protection of arsenic-induced hepatic disorder by arjunolic acid. Basic Clin Pharmacol Toxicol 101:333–338
Saxena M, Faridi U, Mishra R, Gupta MM, Darokar MP, Srivastava SK et al (2007) Cytotoxic agents from Terminalia arjuna. Planta Med 73:1486–1490
Manna P, Sinha M, Sil PC (2008) Protection of arsenic-induced testicular oxidative stress by arjunolic acid. Redox Rep 13:67–77
Ribeiro IG, Silva KCM, Parrini SC, Miranda ALP, Fraga CAM, Barreiro EJ (1998) The anti-inflammatory and analgesic properties of the extractsand arjunolic acid were evaluated in the carrageenan-inducedrat paw edema (CIRPE) (a), acetic acid-induced mice abdominalconstrictions (b). Eur J Med Chem 33:225
Devasagayam TPA, Boloor KK (2003) Methods for estimating lipid peroxidation: an analysis of merits and demerits. Indian J Biochem Biophys 40:300–308
Kumar A (2012) Effect of simuastation on paraxonase 1 (PON1) activity and oxidation stress. In: Kumar A (ed) Significance of lipid profile assay as diagnostic and prognostic tool. Create space independent publishing platform, California. pp 105–109
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase, and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5:51–66
Lewis A, Du J, Liu J, Ritchie JM, Oberley LW, Cullen JJ (2006) Metastatic progression of pancreatic cancer: changes in antioxidant enzymes and cell growth. Clin Exp Metastasis 22:523–532
Liu J, Du J, Zhang Y, Sun W, Smith BJ, Oberley LW et al (2006) Suppression of the malignant phenotype in pancreatic cancer by the overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum Gene Ther 17:105–116
Singh S, Mondal P, Trigun SK (2014) Acute liver failure in rats activates glutamine-glutamate cycle but declines antioxidant enzymes to induce oxidative stress in cerebral cortex and cerebellum. PLoS One 9(4):e95855
Brandt RB, Laux JE, Spainhour SE, Kline ES (1987) Lactate dehydrogenase in rat mitochondria. Arch Biochem Biophys 259:412–422
Paoletti F, Mocali A, Aldinucci D (1990) Superoxide-driven NAD(P)H oxidation induced by EDTA manganese complex and mercapto ethanol. Chem Biol Interact 76:3–18
Sing NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cells Res 175:184–191
Talalay P (1962) Hydroxysteroid dehydrogenase. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic, New York, pp 512–516
Jarabak J, Adams JA, Williams-Ashman HG, Talalay P (1962) Purification of 17β-hydroxysteroid dehydrogenase function. J Biol Chem 237:345–357
Stefova M, Stafilov T, Stojanoski K, Cepreganova-Krstic B (1997) Determination of vitamin B12 in multivitamin tablets by high performance liquid chromatography. Anal Lett 30:2723–2731
De Leenheer AP, Lambert WE, Van Bocxlaer JF (2000) Modern chromatographic analysis of vitamins, 3rd Edition, Revised and Expanded edn. Marcel Dekker, New York ISBN 0-8247-0316-2
Lee DS, Griffith BW (1985) Human serum vitamin B12 assay methods—a review. Clin Biochem 18:261–266
Kalmbach R, Paul L, Selhub J (2011) Determination of unmetabolized folic acid in human plasma using affinity HPLC. Am J Clin Nutr 94:343S–347S
Robitaille L, John L (2015) A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr J 15:40
Zar JH (1996) One sample hypothesis. In: Zar JH (ed) Biostatistical analysis. Prentice Hall, Upper Saddle River, pp 93–98
Wang L, Xu ZR, Jia XY, Jiang JF, Han XY (2006) Effects of arsenic, As (III) on lipid peroxidation, glutathione content and antioxidant enzymes in growing pigs. Asian Aust J Anim Sci 19:727–733
Wnek SM, Kuhlman CL, Camarillo JM, Medeiros MK, Liu KJ, Lau SS et al (2011) Interdependent genotoxic mechanisms of monomethylarsonous acid, role of ROS-induced DNA damage and poly (ADP-ribose) polymerase-1 inhibition in the malignant transformation of urothelial cells. Toxicol Appl Pharmacol 15:1–13
Ciaccio PJ, Gicquel E, O'Neill PJ, Scribner HE, Vandenberghe YL (1998) Investigation of the positive response of ethyl acrylate in the mouse lymphoma genotoxicity assay. Toxicol Sci 46:324–332
Kligerman AD, Doerr CL, Tennant AH, Harrington-Brock K, Allen JW, Winkfield E et al (2003) Methylated trivalent arsenicals as candidate ultimate genotoxic forms of arsenic, induction of chromosomal mutations but not gene mutations. Environ Mol Mutagen 42:192–205
Liu SX, Athar M, Lippai I, Waldren C, Hei TK (2001) Induction of oxyradicals by arsenic: implication for mechanism of genotoxicity. Proc Natl Acad Sci U S A 98:1643–1648
Salnikow K, Zhitkovich A (2008) Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis, nickel, arsenic, and chromium. Chem Res Toxicol 21:28–44
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D et al Arsenic toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
Rydberg B, Lindahl T (1982) Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction. EMBO J 1:211–216
Faraci FM, Didion SP (2004) Vascular protection, superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24:1367–1373
Zhang TC, Schmitt MT, Mumford JL (2003) Effects of arsenic on telomerase and telomeres in relation to cell proliferation and apoptosis in human keratinocytes and leukemia cells in vitro. Carcinogenesis 24:1811–1817
Fang F, Zhang GY, Gao TL, Xiao WN, Liu YX, Liu Y et al (2013) Changes in the antioxidant level, cell cycle progression and apoptosis of testicular cells in rats with diet-induced impaired glucose regulation. Zhonghua Nan KeXue 19:403–408
Calatayud M, Devesa V, Velez D (2013) Differential toxicity and gene expression in Caco-2 cells exposed to arsenic species. Toxicol Lett 218:70–80
Kim JW, Dang CV (2006) Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res 66:8927–8930
Zhang J, Yao YH, Li BG, Yang Q, Zhang PY, Wang HT (2015) Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors, a systematic review and meta-analysis. Sci Rep 5:9800
Gimeno AL, Goldraij A, Gimeno MF (1979) Effects of aresenite and oxamate on the in vitro functional activity of estrogen-dominated rate uterine horns. Experientia 35:221–222
Acharyya N, Deb B, Chattopadhyay S, Maiti S (2015) Arsenic-induced antioxidant depletion, oxidative DNA breakage, and tissue damages are prevented by the combined action of folate and vitamin B12. Biol Trace Elem 168:122–132
Leppert PC, Jayes FL, Segars JH (2014) The extracellular matrix contributes to mechanotransduction in uterine fibroids. Obstet Gynecol Int 2014:783289. doi:10.1155/2014/783289
Zhang Z, Pratheeshkumar P, Budhraja A, Son YO, Kim D, Shi X (2015) Role of reactive oxygen species in arsenic-induced transformation of human lung bronchial epithelial (BEAS-2B) cells. Biochem Biophys Res Commun 456(2):643–648
Do HM, Hong JK, Jung HW, Kim SH, Ham JH, Hwang BK (2003) Expression of peroxidase-like genes, H2O2 production, and peroxidase activity during the hypersensitive response to Xanthomonas campestris pv. vesicatoria in Capsicum annuum. Mol Plant Microb Interact 16:196–205
DeSombre ER, Lyttle CR (1979) Steroid hormone regulations of uterine peroxidase activity. Adv Exp Med Biol 117:157–171
Hundal HS, Kumar R, Singh K, Singh D (2007) Occurrence and geochemistry of arsenic in groundwater of Punjab. Northwest India Commun Soil Sci Plan 38:2257–2277
Bhatt SM (2012) Biotechnological approaches as an alternative for bioremediation of contaminated ground water table around the globe. J Bioremed Biodegrad 3:5
Chattopadhyay S, Ghosh D (2010) The involvement of hypophyseal-gonadal and hypophyseal-adrenal axes in arsenic-mediated ovarian and uterine toxicity, modulation by hCG. J Biochem Mol Toxicol 24:29–41
Chattopadhyay S, Deb B, Maiti S (2012) Hepatoprotective role of vitamin B12 and folic acid in arsenic intoxicated rats. Drug Chem Toxicol 35(1):81–88
Bhattacharya S, Haldar PK (2013) Trichosanthes dioica root alleviates arsenic induced myocardial toxicity in rats. J Environ Pathol Toxicol Oncol 32(3):251–261
Shi H, Shi X, Liu KJ (2004) Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol Cell Biochem 255:67–78
Goebel HH, Schmidt PE, Bohl J, Tettenborn B, Kramer G, Guttman L (1990) Polyneuropathy due to arsenic intoxication: biopsy studies. J Neuropathol Exp Neurol 49:137–149
Giberson A, Vaziri ND, Mirahamadi K, Rosen S (1976) Hemodialysis of acute arsenic intoxication with transient renal failure. Arch Intern Med 136:1303–1304
Chatterjee A, Chatterji U (2010) Arsenic abrogates the estrogen-signaling pathway in the rat uterus. Reprod Biol Endocrinol 8:80
Hoffbrand AV, Kremenchuzky S, Butterworth ML, Mollin MB (1966) Serum lactic dehydrogenase activity and folate deficiency in myelosclerosis and other haematological diseases. Brit med J 1:577–581
Keskin EY, Keskin M (2015) Severe vitamin B12 deficiency in a 15-year-old boy, presentation with haemolysis and pancytopenia. BMJ Case Rep. doi:10.1136/bcr-2015-209718
Akram Z, Jalali S, Shami SA, Ahmad L, Batool S, Kalsoom O (2010) Adverse effects of arsenic exposure on uterine function and structure in female rat. Exptl Toxicol Pathol 62:451–459
Khorasani H, Zheng Z, Nguyen C, Zara J, Zhang X, Wang J et al (2011) A quantitative approach to scar analysis. Am J Pathol 178:21–28
Chattopadhyay S, Pal Ghosh S, Ghosh D, Debnath J (2003) Effect of dietary co-administration of sodium selenite on sodium arsenite-induced ovarian and uterine disorders in mature albino rats. Toxicol Sci 75:412–422
Singh RKR, Li D, Webb R, Sinclair KD (2009) B-vitamin and homocysteine status determines ovarian response to gonadotropin treatment in sheep. Biol Reprod 80:743–752
Ocal P, Ersoylu B, Cepni I, Guralp O, Atakul N, Irez T, Idil M (2012) The association between homocysteine in the follicular fluid with embryo quality and pregnancy rate in assisted reproduc tive techniques. J Assist Reprod Genet 29:299–304
Berker B, Kaya C, Aytac R, Satiroglu H (2009) Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum Reprod 24(9):2293–2302
Maleedhu P, Vijayabhaskar M, Sharma SSB, Praveen KK, Vasundhara Devi D (2014) Status of homocysteine in polycystic ovary syndrome (PCOS). J Clin Diagn Res 8(2):31–33
Corona G, Toffoli G, Fabris M, Viel A, Zarrelli A, Donada C, Boiocchi M (1997) Homocysteine accumulation in human ovarian carcinoma ascitic/cystic fluids possibly caused by metabolic alteration of the methionine cycle in ovarian carcinoma cells. Eur J Cancer 33(8):1284–1290
Korhonen S, Romppanen EL, Hiltunen M et al (2003) Two exonic single nucleotide polymorphisms in the microsomal epoxide hydrolase gene are associated with polycystic ovary syndrome. Fertil Steril 79(6):1353–1357
Jayarajah M (2005) Homocysteine and cardiovascular disease. In: Rao GHR (ed) Coronary artery disease, risk promoters, pathophysiology and prevention. Jaypee Brothers Medical Publishers P. Ltd., New Delhi, pp 141–149
Jeremy Marcus MD, Mark J, Sarnak MD, Vandana Menon MD (2007) Homocysteine lowering and cardiovascular disease risk: lost in translation. Can J Cardiol 23(9):707–710
Kilicdag EB, Bagis T, Tarim E, Aslan E, Erkanli S, Simsek E, Haydardedeoglu B, Kuscu E (2005) Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Hum Reprod 20(6):1521–1528
Gourgari E, Nella AA, Lodish M, Stratakis CA, Yanovski JA (2014) Case report: vitamin B12 deficiency in an adolescent female with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol 179:254
Kile ML, Ronnenberg AG (2008) Can folate intake reduce arsenic toxicity? Nutr Rev 66:349–353
Hall MN, Liu X, Slavkovich V, Ilievski V, Mi Z, Alam S et al (2009) Influence of cobalamin on arsenic metabolism in Bangladesh. Env Health Perspec 117:1724–1729
Nakamura K (2011) Biomimetic and bio-inspired catalytic system for arsenic detoxification, bio-inspired catalysts with vitamin-B12 cofactor. In: Pramatarova L (ed) On biomimetics. In Tech - Open Access, Rijeka, pp 213–228
Hall MN, Gamble MV (2012) Nutritional manipulation of one-carbon metabolism, effects on arsenic methylation and toxicity. J Toxicol 1-11
Mukherjee S, Das D, Mukherjee M, Das AS, Mitra C (2006) Synergistic effect of folic acid and vitamin B12 in ameliorating arsenic-induced oxidative damage in pancreatic tissue of rat. J Nutr Biochem 5:319–327
Parshad VR, Kaur P, Guraya SS (1990) Reproductive cycles of mammals. In: Saidapur SK (ed) Reproductive cycles of Indian vertebrates. Allied Publishers Ltd, Bombay, pp 347–408
Bennett M (2001) Vitamin B12 deficiency, infertility and recurrent fetal loss. J Reprod Med 46:209–212
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maity, M., Perveen, H., Dash, M. et al. Arjunolic Acid Improves the Serum Level of Vitamin B12 and Folate in the Process of the Attenuation of Arsenic Induced Uterine Oxidative Stress. Biol Trace Elem Res 182, 78–90 (2018). https://doi.org/10.1007/s12011-017-1077-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1077-0