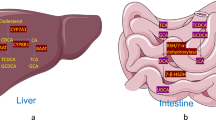
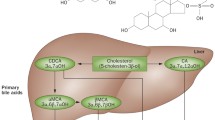
Abstract
Type 2 diabetes (T2D) is a growing health problem worldwide, but the currently available strategies for therapy and prevention are insufficient. Recent observations indicate that bile acid homeostasis is altered in T2D. Bile acids are metabolic regulators that act as signaling molecules through receptor-dependent and -independent pathways. The most prominent signaling molecules mediating bile acid signaling are the nuclear receptor farnesoid X receptor (FXR) and the membrane receptor TGR5. Both are implicated in the regulation of lipid, glucose, and energy metabolism. Dysregulation of these pathways might contribute to the development of T2D and associated complications. Interestingly, data from studies with bile acids or bile acid sequestrants indicate that the manipulation of bile acid homeostasis might be an attractive approach for T2D therapy. In this review, we summarize the mechanisms of bile acid–mediated metabolic control that might be relevant in the pathogenesis of T2D.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
IDF Diabetes Atlas. Available at http://www.diabetesatlas.org/. Accessed January 2011.
Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–91.
Thomas C, Pellicciari R, Pruzanski M, et al. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–93.
Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–74.
Bennion LJ, Grundy SM. Effects of diabetes mellitus on cholesterol metabolism in man. N Engl J Med. 1977;296:1365–71.
Abrams JJ, Ginsberg H, Grundy SM. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes. 1982;31:903–10.
Brufau G, Stellaard F, Prado K, et al. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455–64.
Brufau G, Bahr MJ, Staels B, et al. Plasma bile acids are not associated with energy metabolism in humans. Nutr Metab. 2010;7:73.
Suhre K, Meisinger C, Döring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS ONE. 2010;5:e13953.
Hyogo H, Roy S, Paigen B, et al. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J Biol Chem. 2002;277:34117–24.
• Herrema H, Meissner M, van Dijk TH et al. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor-and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology. 2010; 51:806–816. This study is the first to give detailed insight into bile acid kinetics after sequestration in diabetic animal models.
• Kohli R, Kirby M, Setchell KDR et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G652–60. This paper elegantly shows that short-circuiting of bile acid cycling might contribute to the metabolic improvements observed after bariatric surgery.
Patti M, Houten SM, Bianco AC, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–7.
Li T, Owsley E, Matozel M, et al. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology. 2010;52:678–90.
Prawitt J, Staels B. Bile acid sequestrants: glucose-lowering mechanisms. Metab Syndr Relat Disord. 2010;8 Suppl 1:S3–8.
Schwartz SL, Lai Y, Xu J, et al. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab Syndr Relat Disord. 2010;8:179–88.
Kobayashi M, Ikegami H, Fujisawa T, et al. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes. 2007;56:239–47.
Shang Q, Saumoy M, Holst JJ, et al. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–24.
Chen L, McNulty J, Anderson D, et al. Cholestyramine reverses hyperglycemia and enhances GLP-1 release in Zucker Diabetic Fatty rats. J Pharmacol Exp Ther. 2010;334:164–70.
Prawitt J, Caron S, Staels B. How to modulate FXR activity to treat the metabolic syndrome. Drug Discov Today Dis Mech. 2009;6:e55–64.
Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–25.
Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40.
Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR). Biochem Biophys Res Commun. 2002;298:714–9.
Nguyen A, Bouscarel B. Bile acids and signal transduction: role in glucose homeostasis. Cell Signal. 2008;20:2180–97.
Duran-Sandoval D, Mautino G, Martin G, et al. Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes. 2004;53:890–8.
Duran-Sandoval D, Cariou B, Percevault F, et al. The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition. J Biol Chem. 2005;280:29971–9.
Yamagata K, Daitoku H, Shimamoto Y, et al. Bile acids regulate gluconeogenic gene expression via small heterodimer partner-mediated repression of hepatocyte nuclear factor 4 and Foxo1. J Biol Chem. 2004;279:23158–65.
Ma K, Saha PK, Chan L, et al. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–9.
Stayrook KR, Bramlett KS, Savkur RS, et al. Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 2005;146:984–91.
Zhang Y, Lee FY, Barrera G, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–11.
Cariou B, van Harmelen K, Duran-Sandoval D, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–49.
Cipriani S, Mencarelli A, Palladino G, et al. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–84.
Popescu IR, Helleboid-Chapman A, Lucas A, et al. The nuclear receptor FXR is expressed in pancreatic beta-cells and protects human islets from lipotoxicity. FEBS Lett. 2010;584:2845–51.
Renga B, Mencarelli A, Vavassori P, et al. The bile acid sensor FXR regulates insulin transcription and secretion. Biochim Biophys Acta. 2010;1802:363–72.
Katsuma SEA. Bile acids promote glucagon-like peptide-1 secretion through tgr5 in a murine enteroendocrine cell line stc-1. Biochem Biophys Res Commun. 2005;329:386–90.
Sato H, Genet C, Strehle A, et al. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem Biophys Res Commun. 2007;362:793–8.
• Thomas C, Gioiello A, Noriega L et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009; 10:167–177. This elaborate study is the first to propose a mechanism by which bile acids might improve glucose homeostasis via TGR5.
Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40.
• Kars M, Yang L, Gregor MF et al. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010; 59:1899–1905. This is one of the rare investigations of receptor-independent effects of bile acids on insulin sensitivity in human patients.
Rizzo G, Disante M, Mencarelli A, et al. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Mol Pharmacol. 2006;70:1164–73.
Abdelkarim M, Caron S, Duhem C, et al. The Farnesoid X Receptor Regulates Adipocyte Differentiation and Function by Promoting Peroxisome Proliferator-activated Receptor-{gamma} and Interfering with the Wnt/{beta}-Catenin Pathways. J Biol Chem. 2010;285:36759–67.
Fu L, John LM, Adams SH, et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology. 2004;145:2594–603.
Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–9.
Maruyama T, Tanaka K, Suzuki J, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205.
López M, Varela L, Vázquez MJ, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–8.
Lambert G, Amar MJA, Guo G, et al. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–70.
Bateson MC, Maclean D, Evans JR, et al. Chenodeoxycholic acid therapy for hypertriglyceridaemia in men. Br J Clin Pharmacol. 1978;5:249–54.
Watanabe M, Houten SM, Wang L, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–18.
Kotronen A, Yki-Järvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28:27–38.
Volynets V, Spruss A, Kanuri G, et al. Protective effect of bile acids on the onset of fructose-induced hepatic steatosis in mice. J Lipid Res. 2010;51:3414–24.
Acknowledgments
The authors are supported by the EU Grant HEPADIP (N° 018734) and received funding from Daiichi Sankyo.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prawitt, J., Caron, S. & Staels, B. Bile Acid Metabolism and the Pathogenesis of Type 2 Diabetes. Curr Diab Rep 11, 160–166 (2011). https://doi.org/10.1007/s11892-011-0187-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11892-011-0187-x