Abstract
Objectives
Although transcatheter aortic valve replacement (TAVR) is an excellent alternative procedure for high-risk patients with severe symptomatic aortic stenosis, it is often associated with life-threatening complications. We report on the emergency or elective use of veno-arterial extracorporeal membrane oxygenation (ECMO) to manage these complications.
Methods
Between December 2013 and February 2016, 46 patients underwent TAVR at our institution. Of these, 4 patients required emergency ECMO support and another 3 patients were electively placed on ECMO support at the start of the procedure. The mean age of the ECMO patients was 87.3 ± 3.6 years and all were female. The Society of Thoracic Surgeons-predicted risk of mortality score in these patients was 12.2 ± 6.2%.
Results
TAVR with ECMO was completed through the transapical approach in 6 patients, and the transfemoral approach in 1 patient. The arterial access route for ECMO was the femoral artery in 5, the external iliac artery in 1, and the subclavian artery in 1. Indications for the use of emergency ECMO were hemodynamic instability in 2, cardiogenic shock in 2, while indications for elective ECMO were severe pulmonary hypertension, impaired left ventricular function and a combination of these. There was no 30-day mortality, and the 1-year survival rate was 83.3% with no significant difference compared to patients without ECMO support.
Conclusion
The use of ECMO in very high-risk patients undergoing TAVR may increase safety and contribute to excellent outcomes. Although ECMO support is rarely needed in TAVR, a well-prepared treatment strategy by the heart team is mandatory.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcatheter aortic valve replacement (TAVR) has become an alternative procedure for patients with severe symptomatic aortic stenosis (AS) who are at too high risk for open surgical aortic valve replacement (AVR) [1–7]. Although several randomized trials have shown favorable outcomes for TAVR compared to those of surgical AVR, TAVR carries the risk of serious life-threatening complications [4–7]. A major determinant of early results is the incidence of intraoperative complications such as hemodynamic instability or cardiogenic shock. To reduce and manage these complications, some authors have explored the use of extracorporeal membrane oxygenation (ECMO) [8–12]. The aim of this study was to analyze our initial experiences with patients undergoing TAVR with or without the use of ECMO support at our institution.
Subjects and methods
Patients
Since we started the TAVR program at our institution, a total of 46 patients have undergone TAVR for severe symptomatic AS between December 2013 and February 2016. All the patients were fully screened by our well-organized local heart team. It was determined whether they were at high risk for serious complications if they underwent open surgical AVR. An Edwards SAPIEN XT valve (Edwards Lifesciences, Irvine, Ca, USA) was implanted in all 46 patients. Among these patients, veno-arterial ECMO was used in a total of 7 patients (15%, ECMO group). Of these, 4 required emergency ECMO and 3 received elective ECMO support. These patients were compared to the patients who did not receive ECMO (non-ECMO group). Clinical data were prospectively collected and this study was approved by the institutional review board of Kyoto University Graduate School of Medicine (R0009). Written informed consent was obtained from all the patients and their families.
Evaluation of potential use for ECMO
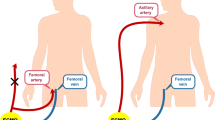
Before TAVR, the heart team, consisting of cardiovascular surgeons, cardiologists, anesthesiologists, nurses and perfusionists, evaluated the risks of possible complications for every single case. Not only the access route for TAVR, but the potential cannulation sites for ECMO (even in patients thought to be at low risk for requiring ECMO during the procedure) were also discussed. Potential arterial cannulation sites included the femoral artery, the iliac artery, and the subclavian artery, depending on the patient’s vascular anatomy. The venous drainage cannula was always inserted from the right femoral vein and advanced into the right atrium.
Emergency use of ECMO
When sudden decrease of systemic blood pressure, elevation of pulmonary artery pressure or ventricular fibrillation were detected before the valve deployment, arterial and venous cannulas were inserted through the already exposed peripheral vessels and ECMO support was initiated immediately to stabilize the hemodynamic status.
Elective use of ECMO
We routinely performed right heart catheterization as well as coronary angiography before the TAVR procedure. In patients with severe pulmonary hypertension (over 60 mmHg) and/or markedly decreased left ventricular ejection fraction (LVEF under 20%), we considered elective ECMO use during the procedure. Occasionally, the access routes for ECMO were surgically exposed even before the induction of general anesthesia in extremely high-risk patients.
Statistical analysis
Continuous variables obtained in this study are expressed as the mean ± standard deviation. Statistical analyses were performed with JMP software version 12 (SAS Institute, Cary, NC, USA). To compare parameters between the two groups, the Student’s t test was used for paired data, and the Mann–Whitney U-test was used for nonparametric data. Survival analysis was performed using the Kaplan–Meier method. A log-rank test was applied for comparisons between time-related variables.
Results
Patient characteristics
Baseline patient clinical characteristics, echocardiographic and cardiac catheterization variables of both ECMO and non-ECMO patients are summarized in Table 1. All of the ECMO patients were frail and diminutive females. ECMO patients had higher grade of the New York Heart Association (NYHA) Functional Class compared to non-ECMO patients (3.0 ± 0.8 vs 2.4 ± 0.6, p < 0.01). Although there were no significant differences in terms of comorbidities except for hypertension, the Society of Thoracic Surgeons (STS) predicted risk of mortality score was significantly higher in the ECMO group than in non-ECMO group (12.2 ± 6.2 vs 7.5 ± 4.1, p = 0.02). Neither the mean LVEF nor the transaortic gradient differed significantly between two groups, but ECMO patients were more likely to have a smaller aortic valve orifice and had a significantly higher incidence of mitral and tricuspid insufficiency compared to non-ECMO patients (p = 0.01, <0.01, 0.04, respectively). In addition, ECMO patients had significantly higher peak pulmonary artery pressure compared to non-ECMO patients (50.8 ± 19.2 vs 31.0 ± 7.2 mmHg, p < 0.01). Of note, 3 patients (42%) in the ECMO group had severe pulmonary artery hypertension (over 60 mmHg).
Emergency use of ECMO
Among the 46 patients, emergency ECMO was necessary in 4 patients. All of the 4 patients underwent TAVR in the first half series of our 46 patients. The details of the ECMO patients are summarized in Table 2. Emergency ECMO was used because of progressively worsening left ventricular function after pericardiotomy in 2 patients with transapical approach. One had moderate pulmonary hypertension preoperatively, and this was gradually worsening after starting the operation. At the time of pericardiotomy, this patient developed severe pulmonary hypertension with severely depressed LV function, which did not respond to medical treatment. The other patient had depressed left ventricular systolic function preoperatively, and developed profound hypotension after pericardiectomy, which was also difficult to manage medically. These two patients had critical AS with extremely high pressure gradient (peak velocity across the aortic valve of 6.6 and 4.6 m/s, respectively). The other 2 patients required emergency ECMO because they developed cardiogenic shock just after balloon aortic valvuloplasty (BAV). These two patients required cardiopulmonary resuscitation before starting ECMO. In 2 cases in the early series of introducing TAVR at our institution, there were some difficulties upon cannulation because we chose relatively large cannulas for both arterial and venous lines. Thereafter, we used smaller cannulas for the other 5 patients, which made it much easier to establish ECMO. In one patient, angiogram showed significant stenosis of the main trunk of the left coronary artery, then percutaneous coronary intervention (PCI) was performed under ECMO support. An intra-aortic balloon pump was used during the weaning from ECMO in one patient who required cardiopulmonary resuscitation before starting emergency ECMO.
Elective use of ECMO
Reasons for elective ECMO included severe pulmonary hypertension in all 3 patients, and 2 of these patients also had severely impaired LVEF. Elective ECMO was started before BAV in 2 patients and before pericardiotomy in 1 patient with prior history of coronary artery bypass grafting. The mean bypass time for all ECMO patients was 52 ± 30 min (range 23–107), with a mean flow index of 1.8 ± 0.3 L min/m2 (range 1.0–2.2). There were no statistical differences between emergency ECMO and elective ECMO patients for these variables. The longest ECMO support time was 107 min for a patient with the highest STS predicted risk of mortality score of 26%. For this high-risk patient, the cannulas for ECMO were inserted before induction of general anesthesia and ECMO support was started just before the insertion of the guide wire from the opposite femoral artery into the left ventricle. Following TAVR, this patient required PCI for acute embolic occlusion in the left anterior descending artery and the first diagonal artery.
Postoperative outcomes
ECMO patients were more likely to undergo TAVR by the transapical approach (Table 3). The mean procedure time was significantly longer in ECMO patients than in non-ECMO patients (200 ± 32 vs 122 ± 30 min, p < 0.01). There were no significant differences in perioperative complications between both groups. However, one emergency ECMO patient (patient 1 in Table 2) who suffered cardiac arrest and needed cardiopulmonary resuscitation during the cannulation suffered temporary cerebral hypoxia and required prolonged intensive care with mechanical ventilation (Table 4). This patient was eventually transferred to another facility for further rehabilitation without any significant neurological sequelae. The 30-day mortality rate was 0% in all of 46 patients. There was one hospital death in the non-ECMO group from sepsis related to refractory pneumonia on postoperative day 43. This patient had respiratory dysfunction related to a long standing history of pneumoconiosis preoperatively. One elective ECMO patient required temporary hemodialysis for three days due to acute renal failure. There was one patient in each group who needed surgical intervention for bleeding: One elective ECMO patient underwent video-assisted thoracoscopic surgery because of a hemothorax after insertion of a drainage tube in the general ward, and one non-ECMO patient needed re-exploration for bleeding from the chest wall a few hours after TAVR. Repair of the access route was performed for one non-ECMO patient three weeks after TAVR because of chronic dissection on the right femoral artery. The length of hospital stay in ECMO group was significantly longer than that in non-ECMO group (38.0 ± 21.2 vs 20.2 ± 9.3 days, p < 0.01). Both peak and mean transaortic pressure gradients were similar and the degree of paravalvular leakage did not significantly differ between the ECMO group and the non-ECMO group (Table 4).
Median follow-up was 12 months. There were 4 late deaths: 1 ECMO patient died of pneumonia 4 months after TAVR, and 2 non-ECMO patients died 7 months after the surgery because of a brain tumor and liver dysfunction, respectively. Another non-ECMO patient died 15 months after TAVR due to an unknown cause. Survival at 1, 6, and 12 months in patients with ECMO and non-ECMO were 100 vs 100%, 83.3 vs 97.2%, and 83.3 vs 90.0%, respectively (log-rank p = 0.71) (Fig. 1).
Discussion
The effective use of ECMO during TAVR has been described in several articles [8–12]. Although 7 of 46 patients (15%) at our institution received ECMO during TAVR, which was higher than those in other reports, our patients showed excellent outcomes. The reason for the higher rate of ECMO in the present series is that we introduced the use of elective ECMO in patients who were judged to be at a high risk of serious complications during the TAVR procedure. We instituted this approach after we experienced one emergency ECMO patient who required cardiopulmonary resuscitation and suffered cerebral hypoxia. Because all of the emergency ECMO patients were in the early stage of introducing TAVR at our institution, the heart team experience and learning curve obviously reduced the use of emergency ECMO later on.
Emergency ECMO introduction was necessary in 8.7% of our TAVR patients, which was similar to the rates reported in other studies [8, 10, 11]. Emergency ECMO is usually introduced to manage life-threatening complications such as aortic root rupture, ventricular perforation, or hemodynamic collapse [8, 11, 12]. In our series, an obvious reason for impaired left ventricular function is acute severe aortic valve regurgitation after BAV. Another possible cause would relate to irritability on the heart. In our emergency ECMO patients, 2 patients showed impaired left ventricle after pericardiotomy. Even small opening of the pericardium might lead to hemodynamic instability, especially in patients with pulmonary hypertension and/or depressed LV function. The selection of arterial cannulation site is also very important. In fact, many of the patients undergoing TAVR have severe atherosclerotic change in the thoracic and abdominal aorta, which would increase the risk of cerebral infarction related to retrograde perfusion. In addition, those undergoing transapical approach may require alternative cannulation site such as subclavian/axillary artery, which is difficult to manage on emergency basis. To reduce the early mortality in emergency ECMO patients, the establishment of a well-organized heart team is mandatory. The heart team should have a clear plan in case emergency ECMO is required. Before TAVR patients arrive in the operating room, the access site for ECMO and the size of cannulas for both arterial and venous lines should be confirmed among the heart team. This allows the team to respond to any serious complications and initiate ECMO as quickly as possible. The fact that we had such a well-prepared team on hand for all our TAVR cases probably accounted for the 0% of early (30-day) mortality rate in the present series. In 2 of our early cases, we selected regular size cannulas, but it was difficult to use these to cannulate small Japanese females with a mean body surface area of 1.1 m2. Consequently, one of these patients required 9 min of cardiopulmonary resuscitation before we successfully initiated ECMO. As Drews et al. pointed out, the use of the smallest cannulas would reduce not only the time for cannulation but the risk of vascular complications [10]. In fact, our study showed no difference in the pump flow according to the size of cannulas for small patients.
Another advantage of ECMO is that it allows the heart team to spend adequate time to assess the proper size and location of the valve during the procedure. In our experience, there were some patients in whom we decided the size of the valve after BAV. These patients, especially those with severe pulmonary hypertension and/or poor LVEF, may develop hemodynamic instability due to acute aortic insufficiency, which often leads to cardiac arrest. In such cases, ECMO support gives the team enough time to prepare and crimp a suitably sized valve.
Recently, some authors have shown the effectiveness of elective ECMO support during TAVR [10, 11]. Their reasons for elective ECMO included: (1) severely impaired left ventricle function; (2) severe pulmonary hypertension; (3) insufficient distance between the coronary artery ostium and the aortic valve annulus. In 3 patients in our series, ECMO was re-started after weaning because significant bleeding from the left ventricular apex was identified where the main sheath for TAVR had been inserted in one patient and for PCI in other 2 patients. In one of the patients with PCI, there was enough distance between both coronary ostium and aortic valve in the CT measurement; the valve was deployed closely to the left coronary ostium. Another patient who required PCI had a markedly reduced LVEF of 15% and used ECMO for 107 min, which was the longest duration in our series. In this case, cannulation for ECMO was completed before the induction of general anesthesia and all the steps in TAVR procedure were performed under ECMO support. Although the patient was weaned off from ECMO, ST elevation on electrocardiogram was noted and a coronary angiogram revealed obstruction of the left anterior descending artery and the diagonal artery. The left main trunk was intact. ECMO was soon re-started and PCI for both arteries was successfully performed under stable hemodynamic conditions due to ECMO support. We reported this case elsewhere [13].
It must be noted, however, that the ECMO patients in our series required significantly longer hospital stays than non-ECMO patients (38.0 ± 21.2 vs 20.2 ± 9.3 days, p < 0.01). As Unbehaun et al. reported, although the TAVR procedure in extremely high-risk patients can be achieved in a safe and stable manner under ECMO support, ECMO patients who had severe pulmonary hypertension preoperatively required prolonged ventilator support and respiratory rehabilitation as a consequence [9]. Another possible reason for this difference is that the ECMO patients were more likely to undergo TAVR via a transapical approach in our series. Many elderly female Japanese patients are small and have skeletal malformation such as kyphosis, which results in narrow intercostal space. Consequently, when the transapical approach was selected in our series, these patients likely suffered rib fractures in the left chest wall. Therefore, the patients tended to stay in the hospital longer for rehabilitation and pain control. We are currently working on making smaller incisions (less than 5 cm) and trying to avoid rib fracture in the transapical approach, which should shorten hospital stays. As some authors have reported, experience and advancement of devices should reduce procedure-related complications [14, 15].
Limitations
The limitations of this study include the small number of patients and the relatively short follow-up period. Furthermore, the learning curve of the surgical team should be taken into account. Another limitation is that only one commercially available prosthesis was implanted in this study. Other devices may yield different outcomes.
Conclusions
We achieved excellent outcomes for TAVR with or without ECMO support in the present cohort. The use of ECMO in very high-risk patients may increase safety and contribute to favorable outcomes.
References
Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106:3006–8.
Gurvitch R, Wood DA, Tay EL, Leipsic J, Ye J, Lichtenstein SV, et al. Transcatheter aortic valve implantation: durability of clinical and hemodynamic outcomes beyond 3 years in a large patient cohort. Circulation. 2010;122:1319–27.
Thielmann M, Wendt D, Eggebrecht H, Kahlert P, Massoudy P, Kamler M, et al. Transcatheter aortic valve implantation in patients with very high risk for conventional aortic valve replacement. Ann Thorac Surg. 2009;88:1468–74.
Lefèvre T, Kappetein AP, Wolner E, Nataf P, Thomas M, Schächinger V, et al. One year follow-up of the multi-centre European PARTNER transcatheter heart valve study. Eur Heart J. 2011;32:148–57.
Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–95.
Holmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, et al. STS/ACC TVT Registry. Annual outcomes with transcatheter valve Therapy: from the STS/ACCTVT Registry. J Am Coll Cardiol. 2015;66:2813–23.
Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, et al. STS/ACC TVT Registry. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–77.
Roselli EE, Idrees J, Mick S, Kapadia S, Tuzcu M, Svensson LG, et al. Emergency use of cardiopulmonary bypass in complicated transcatheter aortic valve replacement: importance of a heart team approach. J Thorac Cardiovasc Surg. 2014;148:1413–6.
Unbehaun A, Pasic M, Buz S, Dreysse S, Kukucka M, Hetzer R, et al. Transapical aortic valve implantation in patients with severely depressed left ventricular function. J Thorac Cardiovasc Surg. 2014;148:2877–82.
Drews T, Pasic M, Buz S, D’Ancona G, Mladenow A, Hetzer R, et al. Elective femoro-femoral cardiopulmonary bypass during transcatheter aortic valve implantation: a useful tool. J Thorac Cardiovasc Surg. 2013;145:757–63.
Husser O, Holzamer A, Philipp A, Nunez J, Bodi V, Müller T, et al. Emergency and prophylactic use of miniaturized veno-arterial extracorporeal membrane oxygenation in transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2013;82:E542–E51.
Negi SI, Patel J, Patel M, Loyalka P, Kar B, Gregoric I. Successful repair of aortic annulus rupture during transcatheter aortic valve replacement using extracorporeal membrane oxygenation support. Gen. Thorac Cardiovasc Surg. 2015;63:514–7.
Tsujimura A, Saito N, Minakata K, Kimura T. Distal coronary embolisation during transcatheter aortic valve implantation. BMJ Case Rep. 2016. doi:10.1136/bcr-2016-216620.
Gurvitch R, Tay EL, Wijesinghe N, Ye J, Nietlispach F, Wood DA, et al. Transcatheter aortic valve implantation: lessons from the learning curve of the first 270 high-risk patients. Catheter Cardiovasc Interv. 2011;78:977–84.
Alli OO, Booker JD, Lennon RJ, Greason KL, Rihal CS, Holmes DR Jr. Transcatheter aortic valve implantation: assessing the learning curve. JACC Cardiovasc Interv. 2012;5:72–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Uehara, K., Minakata, K., Saito, N. et al. Use of extracorporeal membrane oxygenation in complicated transcatheter aortic valve replacement. Gen Thorac Cardiovasc Surg 65, 329–336 (2017). https://doi.org/10.1007/s11748-017-0757-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-017-0757-1