Abstract
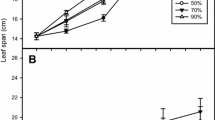
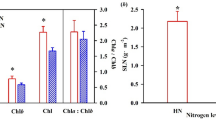
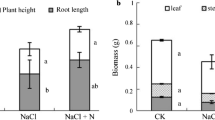
In this study, we used poplar as a model plant and investigated the effects of gaseous nitrogen dioxide (NO2, 4 µl 1−1) on stomatal conductance, photosynthesis, dark- and photorespiration of Populus alba × Populus berolinensis hybrid leaves using the photosynthesis system and scanning electron microscope technique. The results showed that net photosynthetic rates were significantly reduced in leaves exposed to 4 µl 1−1 NO2 for 48 h as compared with leaves exposed to ambient carbon dioxide 380 µl 1−1 and ambient NO2 <0.1 µl 1−1 (the control) and the leaves exposed for 14 h. The decline of net photosynthetic rate was caused mainly by NO2 treatment. Dark respiration rates were dependent on co-action of the two factors (leaf temperature and NO2 treatment time). Post-illumination carbon dioxide burst in the exposed leaves occurred at 13–15 s after turning the light off, whereas this phenomenon was absent in the control leaves.




Similar content being viewed by others
References
Azcon-Bieto J, Osmond CB (1983) Relationship between photosynthesis and respiration: the effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiol 71:574–581
Breuninger C, Meixner FX, Kesselmeier J (2013) Field investigations of nitrogen dioxide (NO2) exchange between plants and the atmosphere. Atmos Chem Phys 13:773–790
Carlson RW (1983) Interaction between SO2 and NO2 and their effects on photosynthetic properties of soybean Glycine max. Environ Pollut Ecol Biol 32:11–38
Chaparro-Suarez IG, Thielmann A, Meixner FX, Kesselmeier J (2006) Re-investigation of the nitrogen dioxide (NO2) uptake by tree species. Geophys Res Abstr 8:706–716
Chaparro-Suarez IG, Meixner FX, Kesselmeier J (2011) Nitrogen dioxide (NO2) uptake by vegetation controlled by atmospheric concentrations and plant stomatal aperture. Atmos Environ 45:5742–5750
Decker JP (1955) A rapid, post-illumination deceleration of respiration in green leaves. Plant Physiol 30:82–84
Dochinger LS, Jensen KF (1985) Effect of acid mist and air pollutants on yellow-poplar seedling height and leaf growth. Research Paper. NE-572. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Broomall, 4 p
Eastham AM, Ormrod DP (1986) Visible injury and growth responses of young cuttings of Populus Canadensis and P. nigra to nitrogen dioxide and sulphur dioxide. Can J Forest Res 16:1289–1292
Eller ASD, McGuire KL, Sparks JP (2011) Responses of sugar maple and hemlock seedlings to elevated carbon dioxide under altered above- and belowground nitrogen sources. Tree Physiol 31:391–401
Furukawa A (1991) Inhibition of photosynthesis of Populus euramericana and Helianthus annuus by SO2, NO2, O3. Ecol Res 6:79–86
Geβler A, Rienks M, Rennenberg H (2000) NH3 and NO2 fluxes between beech trees and the atmosphere—correlation with climatic and physiological parameters. New Phytol 147:539–560
Holopainen T, Anttonen S, Wulff A, Palomäki V, Kärentampi L (1992) Comparative evaluation of effects of gaseous pollutants, acidic deposition and mineral deficiencies: structural changes in the cells of forest plants. Agric Ecosyst Environ 42:365–398
Hu YB, Sun GY (2010) Leaf nitrogen dioxide uptake coupling apoplastic chemistry, carbon/sulfur assimilation, and plant nitrogen status. Plant Cell Rep 29:1069–1077
Hu YB, Bellaloui N, Sun GY, Tigabu M, Wang JH (2014a) Exogenous sodium sulfide improves morphological and physiological responses of a hybrid Populus species to nitrogen dioxide. J Plant Physiol 171:868–875
Hu YB, Fernández V, Ma L (2014b) Nitrate transporters in leaves and their potential roles in foliar uptake of nitrogen dioxide. Front Plant Sci 5:360. doi:10.3389/fpls.2014.00360
Jetter R, Riederer M, Lendzian KJ (1996) The effects of dry O3, SO2 and NO2 on reconstituted epicuticular wax tubules. New Phytol 133:207–216
Kaše M, Čatskŷ J (1983) Post-illumination burst of carbon dioxide in Phaseolus vulgaris L. as affected by leaf temperature. Biol Plant 25:225–230
Kondo K, Yamada K, Nakagawa A, Takahashi M, Morikawa H, Sakamoto A (2008) Molecular characterization of atmospheric NO2-responsive germin-like proteins in azalea leaves. Biochem Biophys Res Commun 377:857–861
Laisk A, Sumberg A (1994) Partitioning of the leaf CO2 exchange into components using CO2 exchange and fluorescence measurements. Plant Physiol 106:689–695
Layne DR, Flore JA, Hanover JW, Mebrahtu T (1991) Leaf temperature effects on net photosynthesis, dark respiration, and photorespiration of seedlings of black locust families with contrasting growth rates. Can J For Res 21:161
Lendzian KJ, Kerstiens G (1988) Interactions between plant cuticles and gaseous air pollutants. Asp Appl Biol 17:97–104
Lin JX, Jach ME, Ceulemans R (2001) Stomatal density and needle anatomy of Scots pine (Pinus sylvestris) are affected by elevated CO2. New Phytol 150:665–674
Mazarura U (2012) Effect of sequences of ozone and nitrogen dioxide on plant dry matter and stomatal diffusive resistance in radish. Afr Crop Sci J 20:371–384
Morikawa H, Higaki A, Nohno M, Takahashi M, Kamada M, Nakata M, Toyohara G, Okamura Y, Matsui K, Kitani S, Fujita K, Irifune K, Goshima N (1998) More than a 600-fold variation in nitrogen dioxide assimilation among 217 plant taxa. Plant Cell Environ 21:180–190
Okano K, Machida T, Totsuka T (1989) Differences in ability of NO2 absorption in various broad-leaved tree species. Environ Pollut 58:1–17
Oleksyn J (1984) Effects of SO2, HF and NO2 on net photosynthetic and dark respiration rates of Scots pine needles of various ages. Photosynthetica 18:259–262
Pallardy SG (2007) Physiology of Woody Plants, 3rd edn. Academic Press, San Diego 480
Parys E, Romanowska E (2000) Relationship between postillumination burst of CO2 and enhancement of respiration in tall fescue leaves. Acta Physiol Plant 22:135–142
Ramge P, Badeck FW, Plöchl M, Kohlmaier GH (1993) Apoplastic antioxidants as decisive elimination factors within the uptake process of nitrogen dioxide into leaf tissues. New Phytol 125:771–785
Rantanen L, Palomakp V, Harrison AF, Lucas PW, Mansfield TA (1994) Interactions between combined exposure to SO2 and NO2 and nutrient status of trees: effects on nutrient content and uptake, growth, needle ultrastructure and pigments. New Phytol 128:689–701
Rennenberg H, Gessler A (1999) Consequences of N deposition to forest ecosystems-recent results and future research needs. Water Air Soil Pollut 116:47–64
Sabaratnam S, Gupta G, Mulchi C (1988) Nitrogen dioxide effects on photosynthesis in soybean. J Environ Qual 17:143–146
Schiffgens-Gruber A, Lutz C (1992) Ultrastructure of mesophyll cell chloroplasts of spruce needles exposed to O3, SO2 and NO2 alone and in combination. Environ Exp Bot 32:243–254
Schmutz P, Tarjan D, Günthardt-Goerg MS, Matyssek R, Bucher JB (1995) Nitrogen dioxide- a gaseous fertilizer of poplar trees. Phyton 35:219–232
Sharkey TD (1988) Estimating the rate of photorespiration in leaves. Physiol Plant 73:147–152
Siegwolf RTW, Matyssek R, Saurer M, Maurer S, Günthardt-Goerg MS, Schmutz P, Bucher JB (2001) Stable isotope analysis reveals differential effects of soil nitrogen and nitrogen dioxide on the water use efficiency in hybrid poplar leaves. New Phytol 149:233–246
Silim SN, Ryan N, Kubien DS (2010) Temperature responses of photosynthesis and respiration in Populus balsamifera L.: acclimation versus adaptation. Photosynth Res 104:19–30
Sparks JP (2009) Ecological ramifications of the direct foliar uptake of nitrogen. Oecologia 159:1–13
Srivastava HS, Jolliffe PA, Runeckles VC (1974a) Inhibition of gas exchange in bean leaves by NO2. Can J Bot 53:466–474
Srivastava HS, Jolliffe PA, Runeckles VC (1974b) The effects of environmental conditions on the inhibition of leaf gas exchange by NO2. Can J Bot 53:475–482
Takagi M, Gyokusen K (2004) Light and atmospheric pollution affect photosynthesis of street trees in urban environments. Urban For Urban Green 2:167–171
Takahashi M, Higaki A, Nohno M, Kamada M, Okamura U, Matsui K, Kitani S, Morikawa H (2005) Differential assimilation of nitrogen dioxide by 70 taxa of roadside trees at an urban pollution level. Chemosphere 61:633–639
Tjoelker MG, Boratynski A, Bugala W (2007) Biology and Ecology of Norway Spruce. In: Werner A (ed) Effects of Pollutants on needle and wood anatomy. Springer, Netherlands, pp 325–327. ISBN 978-83-60247-62-4
Vallano DM, Selmants PC, Zavaleta ES (2012) Simulated nitrogen deposition enhances the performance of an exotic grass relative to native serpentine grassland competitors. Plant Ecol 213:1015–1026
Van Hove LWA, Bossen ME, Mensink MGJ, Van Kooten O (1992) Physiological effects of a long term exposure to low concentrations of ammonia, nitrogen dioxide, sulfur dioxide on douglas fir (Pseudotsuga menziesii). Physiol Plant 86:559–567
Wellburn AR (1990) Why are atmospheric oxides of nitrogen usually phytotoxic and not alternative fertilizers? New Phytol 115:395–429
Yoneyama T, Sasakawa H (1979) Transformation of atmospheric NO2 absorbed in spinach leaves. Plant Cell Physiol 20:263–266
Acknowledgments
This work was supported by grants from the “Fundamental Research Funds for the Central Universities” (Grant No. 2572014CA24; DL10BB24), ‘National Natural Science Foundation’ (Grant No. 31300506), and The Major project for the Heilongjiang Province Science and Technology Program (GZ13B004). The authors gratefully acknowledged Mr. Thomas D. Dahmer and Dr. Josirley de FC Carvalho for language correction.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z. Miszalski.
Rights and permissions
About this article
Cite this article
Hu, Y., Bellaloui, N., Tigabu, M. et al. Gaseous NO2 effects on stomatal behavior, photosynthesis and respiration of hybrid poplar leaves. Acta Physiol Plant 37, 39 (2015). https://doi.org/10.1007/s11738-014-1749-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-014-1749-8