Abstract
Purpose
This study was done to prospectively assess the repeatability and intra- and interobserver variability of first-pass perfusion with 64-detector-row computed tomography (CT) in non-small-cell lung cancer (NSCLC) with a maximum diameter of up to 8 cm.
Materials and methods
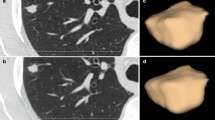
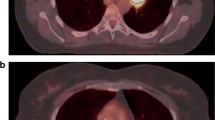
Twelve patients with NSCLC underwent 64-detector-row first-pass CT perfusion (CTP) of the whole tumour. Two different techniques were used according to lesion size (cine mode; sequential mode). After 24 h, each study was repeated to assess repeatability. Lesion blood volume (BV), blood flow (BF), mean transit time (MTT) and peak enhancement intensity (PEI) were automatically calculated by two chest radiologists in two different reading sessions. Intra- and interobserver variability was also assessed.
Results
The first-pass CTP technique was repeatable and precise with within-subject coefficient of variation (WCV) of 9.3, 16.4, 11.2 and 14.9 %, respectively, for BV, BF, MTT and PEI. High intra- and interobserver agreement was demonstrated for each perfusion parameter, with Cronbach’s α coefficients and intraclass correlation coefficients ranging from 0.99 to 1. Precision of measurements was slightly better for intraobserver analysis with WCV ranging between 1.05 and 3.03 %.
Conclusions
Non-small-cell lung cancer first-pass perfusion performed with 64-detector-row CT showed good repeatability and high intra- and interobserver agreement for all perfusion parameters and may be considered a reliable and robust tool for assessing tumour vascularisation.







Similar content being viewed by others
References
Miles KA, Griffiths MR (2003) Perfusion CT: a worthwhile enhancement? Br J Radiol 76:220–231
Horn L, Sandler AB (2009) Angiogenesis in the treatment of non-small cell lung cancer. Proc Am Thorac Soc 6:206–217
Sandler AB, Gray R, Perry MC et al (2006) Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 355:2542–2550
Lind JSW, Meijerink MR, Dingemans AMC et al (2010) Dynamic contrast-enhanced CT in patients treated with sorafenib and erlotinib for non-small cell lung cancer: a new method of monitoring treatment? Eur Radiol 20:2890–2898
Miles KA (2003) Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol 76:S36–S42
Li Y, Yang ZG, Chen TW et al (2008) Peripheral lung carcinoma: correlation of angiogenesis and first-pass perfusion parameters of 64-detector row CT. Lung cancer 61:44–53
Tacelli N, Remy-Jardin M, Copin MC et al (2010) Assessment of non-small cell lung cancer perfusion: pathologic–CT correlation in 15 patients. Radiology 257:863–871
Tateishi U, Nishihara H, Watanabe S et al (2001) Tumor angiogenesis and dynamic CT in lung adenocarcinoma: radiologic-pathologic correlation. J Comput Assist Tomogr 25:23–27
Fraioli F, Anzidei M, Zaccagna F et al (2011) Whole-tumor perfusion CT in patients with advanced lung adenocarcinoma treated with conventional and antiangiogenetic chemotherapy: initial experience. Radiology 259:574–582
Ng QS, Goh V, Carnell D et al (2007) Tumor antivascular effects of radiotherapy combined with combretastatin A4 phosphate in human non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 67:1375–1380
Miles KA, Griffiths MR, Fuentes MA (2001) Standardized perfusion value: universal CT contrast enhancement scale that correlates with FDG PET in lung nodules. Radiology 220:548–553
Ng QS, Goh V, Klotz E et al (2006) Quantitative assessment of lung cancer perfusion using MDCT: does measurement reproducibility improve with greater tumor volume coverage? AJR Am J Roentgenol 187:1079–1084
Ng QS, Goh V, Fichte H et al (2006) Lung cancer perfusion at multi-detector row CT: reproducibility of whole tumor quantitative measurements. Radiology 239:547–553
Prokop M (2003) Radiation dose and image quality. In: Prokop M, Galanski M (eds) Spiral and multislice computed tomography of the body. Georg Thieme Verlag, Stuttgart, pp 131–160
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Bland JM, Altman DG (2003) Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol 22:85–93
Cronbach LJ (1951) Coefficient alpha and the internal structure of tests. Psychometrika 16:297–334
Ng QS, Goh V (2010) Angiogenesis in non-small cell lung cancer. Imaging with perfusion computed tomography. J Thorac Imaging 25:142–150
Chaplin DJ, Hill SA (1995) Temporal heterogeneity in microregional erythrocyte flux in experimental solid tumors. Br J Cancer 71:1210–1213
Kimura H, Braun RD, Ong ET et al (1996) Fluctuations in red cells flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res 56:5522–5528
Ng CS, Chandler AG, Wei W et al (2011) Reproducibility of perfusion parameters obtained from perfusion CT in lung tumors. AJR Am J Roentgenol 197:113–121
Petralia G, Preda L, D’Andrea G et al (2010) CT perfusion in solid-body tumours. Part I: technical issues. Radiol Med 115:843–857
Li Y, Yang ZG, Chen TW et al (2008) Whole tumour perfusion of peripheral lung carcinoma: evaluation with first-pass CT perfusion imaging at 64-detector row CT. Clin Radiol 63:629–635
Goh V, Halligan S, Hugill JA et al (2005) Quantitative assessment of colorectal cancer perfusion using MDCT: inter- and intraobserver agreement. AJR Am J Roentgenol 185:225–231
Conflict of interest
A. R. Larici, L. Calandriello, M. Amato, R. Silvestri, A. del Ciello, F. Molinari, C. de Waure, M. L. Vita, G. Carnassale, and L. Bonomo declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Larici, A.R., Calandriello, L., Amato, M. et al. First-pass perfusion of non-small-cell lung cancer (NSCLC) with 64-detector-row CT: a study of technique repeatability and intra- and interobserver variability. Radiol med 119, 4–12 (2014). https://doi.org/10.1007/s11547-013-0300-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-013-0300-0