Abstract
Histopathological evidence supports the idea that the emergence of phenotypic heterogeneity and resistance to cytotoxic drugs can be considered as a process of selection in tumor cell populations. In this framework, can we explain intra-tumor heterogeneity in terms of selection driven by the local cell environment? Can we overcome the emergence of resistance and favor the eradication of cancer cells by using combination therapies? Bearing these questions in mind, we develop a model describing cell dynamics inside a tumor spheroid under the effects of cytotoxic and cytostatic drugs. Cancer cells are assumed to be structured as a population by two real variables standing for space position and the expression level of a phenotype of resistance to cytotoxic drugs. The model takes explicitly into account the dynamics of resources and anticancer drugs as well as their interactions with the cell population under treatment. We analyze the effects of space structure and combination therapies on phenotypic heterogeneity and chemotherapeutic resistance. Furthermore, we study the efficacy of combined therapy protocols based on constant infusion and bang–bang delivery of cytotoxic and cytostatic drugs.











Similar content being viewed by others
References
Arnold A, Desvillettes L, Prévost C (2012) Existence of nontrivial steady states for populations structured with respect to space and a continuous trait. Commun Pure Appl Anal 11:83–96
Beketic-Oreskovic L, Durán GE, Chen G, Dumontet C, Sikic BI (1995) Decreased mutation rate for cellular resistance to doxorubicin and suppression of MDR1 gene activation by the cyclosporin PSC 833. J Natl Cancer Inst 87:1593–1602
Beyer I, van Rensburg R, Strauss R, Li Z, Wang H, Persson J, Yumul R, Feng Q, Song H, Bartek J et al (2011) Epithelial junction opener JO-1 improves monoclonal antibody therapy of cancer. Cancer Res 71:7080–7090
Beyer I, Cao H, Persson J, Song H, Richter M, Feng Q, Yumul R, van Rensburg R, Li Z, Berenson R et al (2012) Coadministration of epithelial junction opener JO-1 improves the efficacy and safety of chemotherapeutic drugs. Clin Cancer Res 18:3340–3351
Bouin E, Calvez V, Meunier N, Mirrahimi S, Perthame B, Raoul G, Voituriez R (2012) Invasion fronts with variable motility: phenotype selection, spatial sorting and wave acceleration. Comptes Rendus Math 350:761–766
Brodie ED III (1992) Correlational selection for color pattern and antipredator behavior in the garter snake Thamnophis ordinoides. Evolution 46:1284–1298
Busch TM, Xing X, Yu G, Yodh A, Wileyto EP, Wang H-W, Durduran T, Zhu TC, Wang KK-H (2009) Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem Photobiol Sci 8:1683–1693
Byrne H (2013) Continuum models of avascular tumor growth, in mathematics and life sciences. Antoniouk AV, Melnik RVN (eds) De Gruyter, ch. 12.1, pp 279–312
Carver K, Ming X, Juliano RL (2014) Multicellular tumor spheroids as a model for assessing delivery of oligonucleotides in three dimensions. Mol Ther Nucleic Acids 3:e153
Corbett T, Griswold D, Roberts B, Peckham J, Schabel F (1975) Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res 35:2434–2439
de Bruin EC, Taylor TB, Swanton C (2013) Intra-tumor heterogeneity: lessons from microbial evolution and clinical implications. Genome Med 5:101
de Pillis LG, Radunskaya AE, Wiseman CL (2005) A validated mathematical model of cell-mediated immune response to tumor growth. Cancer Res 65:7950–7958
de Pillis L, Renee Fister K, Gu W, Collins C, Daub M, Gross D, Moore J, Preskill B (2009) Mathematical model creation for cancer chemo-immunotherapy. Comput Math Methods Med 10:165–184
De Pillis L, Savage H, Radunskaya A (2013) Mathematical model of colorectal cancer with monoclonal antibody treatments, arXiv preprint, arXiv:1312.3023
Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan MD et al (2012) Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481:506–510
Foo J, Chmielecki J, Pao W, Michor F (2012) Effects of pharmacokinetic processes and varied dosing schedules on the dynamics of acquired resistance to erlotinib in EGFR-mutant lung cancer. J Thorac Oncol 7:1583–1593
Gatenby R (2009) A change of strategy in the war on cancer. Nature 459:508–509
Gatenby R, Silva A, Gillies R, Frieden B (2009) Adaptive therapy. Cancer Res 69:4894–4903
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892
Goldie J, Coldman A (1998) Drug resistance in cancer: mechanisms and models. Cambridge University Press, Cambridge
Gottesman M (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627
Grothey A (2006) Defining the role of panitumumab in colorectal cancer. Community Oncol 3:6–10
Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES (2011) Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 146:633–644
Inukai M, Toyooka S, Ito S, Asano H, Ichihara S, Soh J, Suehisa H, Ouchida M, Aoe K, Aoe M et al (2006) Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res 66:7854–7858
Jackson TL, Byrne HM (2002) A mechanical model of tumor encapsulation and transcapsular spread. Math Biosci 180:307–328
Kimmel M, Świerniak A (2006) Control theory approach to cancer chemotherapy: benefiting from phase dependence and overcoming drug resistance. In: Friedman A (ed)Tutorials in mathematical biosciences III, vol 1872 of lecture notes in mathematics. Springer, Berlin, pp 185–221
Komarova N, Wodarz D (2005) Drug resistance in cancer: principles of emergence and prevention. Proc Natl Acad Sci USA 102:9714–9719
Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, Sougnez C, Stewart C, Sivachenko A, Wang L et al (2013) Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152:714–726
Laurent J, Frongia C, Cazales M, Mondesert O, Ducommun B, Lobjois V (2013) Multicellular tumor spheroid models to explore cell cycle checkpoints in 3d. BMC Cancer 13:1–12
Lavi O, Greene JM, Levy D, Gottesman MM (2013) The role of cell density and intratumoral heterogeneity in multidrug resistance. Cancer Res 73:7168–7175
Łędżewicz U, Schättler H (2002) Optimal bang–bang controls for a two-compartment model in cancer chemotherapy. J Opt Theory Appl 114:609–637
Lorz A, Mirrahimi S, Perthame B (2011) Dirac mass dynamics in a multidimensional nonlocal parabolic equation. Commun Part Differ Equ 36:1071–1098
Lorz A, Lorenzi T, Hochberg ME, Clairambault J, Perthame B (2013) Populational adaptive evolution, chemotherapeutic resistance and multiple anti-cancer therapies. Esaim Math Model Numer Anal 47:377–399
Luria SE, Delbrück M (1943) Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491–511
Merlo L, Pepper J, Reid B, Maley C (2006) Cancer as an evolutionary and ecological process. Nat Rev Cancer 6:924–935
Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer 6:583–592
Mirrahimi S, Perthame B (2014) Asymptotic analysis of a selection model with space (preprint)
Mirrahimi S, Raoul G (2013) Dynamics of sexual populations structured by a space variable and a phenotypical trait. Theor Popul Biol 84:87–103
Mitchison T (2012) The proliferation rate paradox in antimitotic chemotherapy. Mol Biol Cell 23:1–6
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D et al (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324:1457–1461
Perthame B, Barles G (2008) Dirac concentrations in Lotka–Volterra parabolic PDEs. Indiana Univ Math J 57:3275–3301
Rainey PB, Travisano M (1998) Adaptive radiation in a heterogeneous environment. Nature 394:69–72
Scotto K (2003) Transcriptional regulation of abc drug transporters. Oncogene 22:7496–7511
Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, McDermott U, Azizian N, Zou L, Fischbach MA, Wong K-K, Brandstetter K, Wittner B, Ramaswamy S, Classon M, Settleman J (2010) A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 141:69–80
Silva A, Gatenby R (2010) A theoretical quantitative model for evolution of cancer chemotherapy resistance. Biol Direct 22:5–25
Simon R, Norton L (2006) The Norton–Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol 3:406–407
Sutherland RM (1988) Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 240:177–184
Swanton C (2010) Intratumor heterogeneity: evolution through space and time. Cancer Res 72:4875–4882
Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM (2006) Targeting multidrug resistance in cancer. Nat Rev Drug Discov 5:219–234
Tabernero J, Van Cutsem E, Díaz-Rubio E, Cervantes A, Humblet Y, André T, Van Laethem J-L, Soulié P, Casado E, Verslype C, Valera JS, Tortora G, Ciardiello F, Kisker O, de Gramont A (2007) Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 25:5225–5232
Tomasetti C, Levy D (2010a) Drug resistance always depends on the turnover rate. In: Herold K, Vossoughi J, Bentley W (eds) SBEC 2010, IFMBE proceedings, vol 32. Springer, Berlin, pp 552–555
Tomasetti C, Levy D (2010b) An elementary approach to modeling drug resistance in cancer. Math Biosci Eng 7:905–918
Tsai C-J, Nussinov R (2013) The molecular basis of targeting protein kinases in cancer therapeutics. In: Seminars in cancer biology, vol 23. Elsevier, Amsterdam, pp 235–242
Vaupel P, Hockel M (2000) Blood supply, oxygenation status and metabolic micromilieu of breast cancers: characterization and therapeutic relevance. Int J Oncol 17:869–948
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Weinberg R (2007) The biology of cancer. Garland Science, London
Weinstein IB, Joe A (2008) Oncogene addiction. Cancer Res 68:3077–3080
Ye L-C, Liu T-S, Ren L, Wei Y, Zhu D-X, Zai S-Y, Ye Q-H, Yu Y, Xu B, Qin X-Y, Xu J (2013) Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol 31:1931–1938
Yu P, Mustata M, Peng L, Turek JJ, Melloch MR, French PM, Nolte DD (2004) Holographic optical coherence imaging of rat osteogenic sarcoma tumor spheroids. Appl Opt 43:4862–4873
Zhou DC, Ramond S, Viguié F, Faussat AM, Zittoun R, Marie JP (1996) Sequential emergence of MRP- and MDR1-gene over-expression as well as MDR1-gene translocation in homoharringtonine-selected K562 human leukemia cell lines. Int J Cancer 65:365–371
Acknowledgments
The research leading to this paper was (partially) funded by the French “ANR blanche” Project Kibord: ANR-13-BS01-0004. T.L. was supported by the Fondation Sciences Mathématiques de Paris and by a public grant overseen by the French National Research Agency (ANR) as part of the “Investissements d’Avenir” program (reference: ANR-10-LABX-0098), and by the FIRB Project—RBID08PP3J.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alexander Lorz and Tommaso Lorenzi: These primary authors contributed equally to this article.
Appendices
Appendix 1: Qualitative Mathematical Justification for Phenotypic Selection
From a mathematical standpoint, using the considerations drawn in Lorz et al. (2011), Mirrahimi and Perthame (2014) and Perthame and Barles (2008), the long-term dynamics of the concentration points \(X(t,r)\) can be formally characterized by evaluating
In order to verify it numerically, we write the following identity
which implies, due to the definitions of the functions \(p\) and \(\mu _1\) provided in Sect. 3,
Figure 12 shows that the curves \(X(t,r)\) obtained from the formula above for \(t\) equal to the final time of simulations are in good agreement with the positions of the maximum points \(x_M(t,r)\) of \(n(t,r,x)/\rho (t,r)\) at the same time instant.
Qualitative mathematical justification for phenotypic selection. Plots of \(X(t,r)\) (solid lines) and positions of the maximum points \(x_M(t,r)\) of \(n(t,r,x)/\rho (t,r)\) (\(\cdot \)) for \(C_{1,2}(\cdot ) = 0\) and \(t=50\) (left panel); \(C_1(\cdot ) = 0, C_2(\cdot ) = C_2 > 0\) and \(t=200\) (center panel); \(C_1(\cdot ) = C_1 > 0, C_2(\cdot ) = 0\) and \(t=120\) (right panel). The unit of time is days
Appendix 2: Values of the Parameters
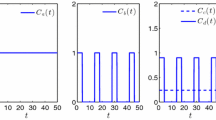
Figure 2: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48\); (a) , \(C_1(\cdot ) = 0, C_2(\cdot ) = 0, \mu _2=800\); (b) \(C_1(\cdot ) = 0, C_2(\cdot ) = 3, \mu _2=8\); (c) \(C_1(\cdot ) = 3, C_2(\cdot ) = 0, \mu _2=800\).
Figure 3: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48\); (left) \(C_1(\cdot ) = 3, C_2(\cdot ) = 0, \mu _2=800\); (right) \(C_1(\cdot ) := 0, C_2(\cdot ) := 3, \mu _2=8\).
Figure 4: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48, \mu _2=8, C_1 = 4, C_2(\cdot ) = 0\).
Figure 5: \(C^0=0.05, \varepsilon =0.025, S_1 = 1.6, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48, \mu _2=800, C_a = 2.4, C_b = 3.6, C_d = 6, C_2(\cdot ) = 0\).
Figure 7: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48, \mu _2=800, C_1(\cdot ) = 0, C_2(\cdot ) = 0\).
Figures 8 and 9: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, =1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48, \mu _2=800, C_a = 4, C_b = 15\).
Figure 10: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48, \mu _2=800, C_a = 0.24, C_b = 0.90\).
Figure 11: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48, \mu _2=800, C_d = 0.24, C_e = 0.90\).
Figure 12: \(C^0=0.005, \varepsilon =0.005, S_1 = 12, \alpha _s=0.08, \alpha _{c_1}=0.08, \alpha _{c_2}=0.2, \gamma _s=1, \gamma _{c_1}=1, \gamma _{c_2}=1, d=14.48\); (left) \(C_1(\cdot ) = 0, C_2(\cdot ) = 0, \mu _2=800\); (center) \(C_1(\cdot ) = 0, C_2(\cdot ) = 3, \mu _2=8\); (right) \(C_1(\cdot ) = 3, C_2(\cdot ) = 0, \mu _2=800\).
Rights and permissions
About this article
Cite this article
Lorz, A., Lorenzi, T., Clairambault, J. et al. Modeling the Effects of Space Structure and Combination Therapies on Phenotypic Heterogeneity and Drug Resistance in Solid Tumors. Bull Math Biol 77, 1–22 (2015). https://doi.org/10.1007/s11538-014-0046-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0046-4