Abstract
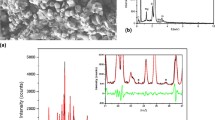
A novel class of solid solutions of Y2−x Sm x W3O12 (x = 0.0−0.4) were synthesized and studied by means of powder X-ray diffraction. All samples crystallize in an orthorhombic space group Pnca. The lattice parameters a, b and c of Y2−x Sm x W3O12 increase with increasing Sm content. Since the compounds of this series hydrate at room temperature, thermogravimetric (TG) analysis was carried out. The result shows that the compound stores less water with increasing Sm content. The thermal expansion properties of Y2−x Sm x W3O12 (x = 0.1, 0.3 and 0.4) were investigated with high temperature X-ray diffraction. Negative thermal expansion coefficient α I becomes less negative from −6.644×10−6 to −6.211×10−6°C−1 when x changes from 0.1 to 0.4.
Similar content being viewed by others
References
Evans J S O, Mary T A, Sleight A W. Negative thermal expansion in a large molybdate and tungstate family. J Solid State Chem, 1997, 133: 580–583
Evans J S O, Mary T A, Sleight A W. Negative thermal expansion in Sc2(WO4)3. J Solid State Chem, 1998, 137: 148–160
Forster P M, Yokochi A, Sleight A W. Enhanced negative thermal expansion in Lu2W3O12. J Solid State Chem, 1998, 140: 157–158
Marinkovic B A, Jardim P M, de Avillez R R, et al. Negative thermal expansion in Y2Mo3O12. Solid State Sci, 2005, 7: 1377–1383
Woodcock D A, Lightfoot P, Ritter C. Negative thermal expansion in Y2(WO4)3. J Solid State Chem, 2000, 149: 92–98
Forster P M, Sleight A W. Negative thermal expansion in Y2W3O12. Inter J Inorg Mater, 1999, 1: 123–127
Sumithra S, Tyagi A K, Umarji A M. Negative thermal expansion in Er2W3O12 and Yb2W3O12 by high temperature X-ray diffraction. Mater Sci Eng B Solid, 2005, 116: 14–18
Sumithra S, Umarji A M. Role of crystal structure on the thermal expansion of Ln2W3O12(Ln=La, Nd, Dy, Y, Er and Yb). Solid State Sci, 2004, 6: 1313–1319
Mary T A, Sleight A W. Bulk thermal expansion for tungstate and molybdates of the type A2M3O12. J Mater Res, 1999, 14(3): 912–915
Varga T, Wilkinson A P, Lind C, et al. High pressure synchrotron x-ray powder diffraction study of Sc2Mo3O12 and Al2W3O12. J Phys: Condens Matter, 2005, 17: 4271–4283
Sumithra S, Umarji A M. Hygroscopicity and bulk thermal expansion in Y2W3O12. Mater Res Bull, 2005, 40: 167–176
Tyagi A K, Achary S N, Mathews M D. Phase transition and negative thermal expansion in A2(MoO4)3 system (A=Fe3+, Cr3+ and Al3+). J Alloy Comp, 2002, 339: 207–210
Gaertner M, Abeln D, Pring A, et al. Synthesis, structure, and reactivity of novel lanthanum tungstates. J Solid State Chem, 1994, 111: 128–133
Templeton D H, Zalkin A. Crystal structure of europium tungstate. Acta Crystallogr, 1963, 16: 762–766
Wu M M, Peng J, Cheng Y Z, et al. Structure and thermal expansion properties of solid solution Nd2−x ErxW3O12 (0.0≤x≤0.6 and 1.5≤x≤2.0). Solid State Sci, 2006, 8: 665–670
Suzuki T, Omote A. Zero thermal expansion in (Al2x (HfMg)1−x )(WO4)3. J Am Ceram Soc, 2006, 89(2): 691–693
Rodriguez-Carvajal J. An Introduction to the Program Fullprof 2000. Version July 2001. Laboratoire Leon Brillouin, France, 2001
Chang L L Y, Scroger M G, Phillips B. High temperature phase equilibria in the systems Sm2O3-WO3 and Sm2O3-W-WO3. J Inorg Nucl Chem, 1966, 28: 1179–1184
Diamond 3.0: Crystal and molecular structure visualization. Version 3.0. 2005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Z., Peng, J., Wang, H. et al. Thermal expansion properties and hygroscopicity of Y2−x Sm x W3O12 (x = 0.0–0.4) compounds. Sci. China Ser. E-Technol. Sci. 51, 25–32 (2008). https://doi.org/10.1007/s11431-007-0040-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11431-007-0040-2