Abstract
Many age-related diseases are associated with, and may be promoted by, cardiac fibrosis. Transforming growth factor (TGF)-β, hypoxia-induced factor (HIF), and the matrix metalloproteinase (MMP) system have been implicated in fibrogenesis. Thus, we investigated whether age is related to these systems and to atrial fibrosis. Right atrial appendages (RAA) obtained during heart surgery (n = 115) were grouped according to patients’ age (<50 years, 51–60 years, 61–70 years, or >70 years). Echocardiographic ejection fractions (EF) and fibrosis using Sirius-red-stained histological sections were determined. TGF-β was determined by quantitative RT-PCR and hypoxia-related factors [HIF1α, the vascular endothelial growth factor (VEGF)-receptor, CD34 (a surrogate marker for microvessel density), the factor inhibiting HIF (FIH), and prolyl hydroxylase 3 (PHD 3)] were detected by immunostaining. MMP-2 and -9 activity were determined zymographically, and mRNA levels of their common tissue inhibitor TIMP-1 were determined by RT-PCR. Younger patients (<50 years) had significantly less fibrosis (10.1% ± 4.4% vs 16.6% ± 8.3%) than older individuals (>70 years). While HIF1α, FIH, the VEGF-receptor, and CD34 were significantly elevated in the young, TGF-β and PHD3 were suppressed in these patients. MMP-2 and -9 activity was found to be higher while TIMP-1 levels were lower in older patients. Statistical analysis proved age to be the only factor influencing fibrogenesis. With increasing age, RAAs develop significantly more fibrosis. An increase of fibrotic and decrease of hypoxic signalling and microvessel density, coupled with differential expression of MMPs and TIMP-1 favouring fibrosis may have helped promote atrial fibrogenesis.
Similar content being viewed by others
Introduction
Many diseases such as hypertension, congestive heart failure, and atrial fibrillation have been linked to aging (Ho et al. 1993; Kannel et al. 1982; Staessen et al. 1999). These conditions are also known to be associated with fibrosis (Brilla 2000b; Gramley et al. 2007; Ohtani et al. 1995). Age has been suggested to play a role during the development of cardiac fibrosis (Annoni et al. 1998; Gazoti Debessa et al. 2001; Goette et al. 2002; Robert et al. 1997). In recent years, ventricular fibrosis has been thoroughly characterised (Brilla 2000a; Khan and Sheppard 2006; Lijnen et al. 2000). These findings cannot simply be transferred to atrial fibrosis and remodelling, as recently shown in animal models (Nakajima et al. 2000; O’Brien et al. 2000). Various culprits have been identified in atrial fibrogenesis. Among these, transforming growth factor (TGF)-β, hypoxia, and the matrix metalloproteinase (MMP)-system may play a role(Gramley et al. 2007).
The TGF-β superfamily includes more than 30 members that participate in development, differentiation, tissue repair, and tumorigenesis, but which also modulate immune and endocrine functions. The TGF-β signalling pathway constitutes a central regulating system in cardiac fibrogenesis (Hao et al. 1999; Li et al. 2008; Lijnen et al. 2000; Seeland et al. 2002; Wang et al. 2002) and may up-regulate pro-angiogenic signals (Li et al. 1997).
Deficient oxygen supply resulting in cardiac remodelling and fibrogenesis is often a cause of myocardial dysfunction (De Boer et al. 2003). However, cardiac remodelling and fibrogenesis may also impair coronary microcirculation (De Boer et al. 2003). Recently, hypoxia and the hypoxia-induced factor (HIF)-pathway have been implicated in age-related renal changes (Tanaka et al. 2006). Furthermore, the HIF pathway has been suggested as being involved in the development of atrial fibrillation (Thijssen et al. 2002). In the absence of oxygen, HIF1 binds to the hypoxia response elements (HRE) and induces its target genes such as the vascular endothelial growth factor (VEGF) (Harris 2002)—which may also be induced by TGF-β (Li et al. 1997). Subsequently, VEGF may interact with its receptors, among which the kinase insert-domain containing receptor (KDR) is the key receptor for transmission of its angiogenic effects (Ferrara et al. 2003). Prolyl hydroxylase 3 (PHD3) is the most prominent of three PHDs in the heart, and can effect the proteasome-mediated degradation of HIF by catalysing the hydroxylation of HIF1α under normoxic conditions. In hypoxia or ischemia, PHD-mediated hydroxylation does not occur, and HIF-1α accumulates, resulting in HIF-mediated gene transcription and increased microvessel density (MVD) (Cioffi et al. 2003; Rohrbach et al. 2005). CD34, a glycoprotein, is expressed on endothelial cells and may be used as a surrogate marker to allow the determination of MVD. FIH is an inhibitor of HIF (FIH = factor inhibiting HIF). Thus, regulation of these factors can modify the hypoxic signal and response.
Matrix-metalloproteinases (MMPs) are endogenous zinc-dependent enzymes that are capable of degrading most components of the extracellular matrix. Their function is tightly regulated by tissue inhibitors of metalloproteinases (TIMPs). The MMP-system is of central importance in cardiac fibrogenesis (Sivasubramanian et al. 2001). Recently, MMPs have been shown to be involved in atrial remodelling in various diseases such as atrial fibrillation, heart failure, and mitral valve disease (Anne et al. 2005; Boixel et al. 2003; Gramley et al. 2007; Mukherjee et al. 2006); however, their involvement and contribution to atrial fibrogenesis during aging is not known (see Fig. 1 for a schematic diagram of these processes).
The black lines represent a simplified diagram of transforming growth factor (TGF-β) and hypoxic signalling in fibro- and angio-genesis. TGF-β has profibrotic effects and may be induced by hypoxia. A direct increase of vascular endothelial growth factor (VEGF) transcription by TGF-β has been proposed. Once hypoxia is present, hypoxia-induced factor (HIF) is increased and may also lead to proangiogenic signalling [e.g. via genes encoding VEGF, kinase insert-domain containing receptor (KDR) and TGF-β]. Prolyl hydroxylase 3 (PHD3) and factor inhibiting HIF (FIH) both work to limit the HIF-response. An increased angiogenic signal and microvessel density (MVD) may in turn limit hypoxia (red-to-green gradients indicate progressive hypoxia). The green lines illustrate the changes of these factors observed in old age as suggested by the present study
Consequently, we investigated the possible existence of an age-dependent link between profibrotic and hypoxic signalling as well as the MMP-system on the one hand, and atrial fibrogenesis on the other.
Materials and methods
Patients
Patients scheduled for routine open heart surgery at the RWTH Aachen University Medical Centre between June 2003 and March 2005 were screened for participation in this study. Individuals with relevant co-morbidities such as atrial fibrillation, malignancies, chronic inflammatory diseases, or acute infections were excluded. A total of 115 consecutive patients gave informed consent in accordance with the local ethics committee. Patients were classified according to age: 19 patients were 50 years old or younger, 27 patients were between 51 and 60 years, 29 patients between 61 and 70 years, and 40 patients were 71 years or older. Please refer to Table 1 for detailed patient characteristics.
Prior to open heart surgery, a standard transthoracic echocardiography was performed to estimate ventricular function and calculate atrial volume (Sanfilippo et al. 1990) as well as to detect other pathologies.
Sample processing
To connect the patient to the heart-and-lung machine in open heart surgery, the right atrial appendage (RAA) needs to be canulated. During canulation, a small part of the RAA is routinely resected. Thus, taking these samples carries no additional risk to the patient. RAAs underwent immediate (within 2 min of surgical removal) dissection. One part was snap-frozen in liquid nitrogen and one part underwent fixation in 10% buffered formalin and was processed for paraffin histology. Frozen samples were stored at −80°C.
Morphometry
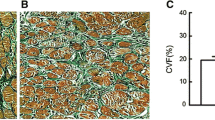
Deparaffined tissue sections (2 μm) were stained with Sirius-red stain. Characteristic areas were photographed using a Zeiss Axioplan 2 imaging microscope (Zeiss, Göttingen, Germany) with a 3200 Kelvin lamp and a Color View II digital camera (Soft Imaging System, Münster, Germany). The photos were then analysed using OpenLab software version 2.2.5 (Improvision, Lexington, MA). To evaluate the amount of mature collagen, Sirius-red stained sections were expressed as percentages of the total tissue area (Fig. 2).
Immunohistochemistry
Sections were dewaxed and rehydrated; antigen retrieval was performed if needed; endogenous peroxidase was blocked using the EnVision System (Dako, Glostrup, Denmark). Sections were developed using diaminobenzidine D5905 (Sigma, Munich, Germany) for 5 min. All immunohistochemistry (IHC) steps were carried out at room temperature. Please see Table 2 for details.
IHC for MVD
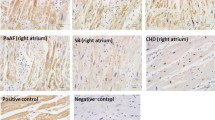
All procedures were performed according to the manufacturer’s instructions for the CSA-Kit K1500 (Dako). The primary antibody (murine monoclonal antibody) CD34 1184 (Immunotech, Marseille, France) was applied undiluted for 60 min at 37°C (see Fig. 3 for a panel of representative stainings).
Histological analysis of IHC
Microphotography was performed as described above. All immunohistochemical stainings were analysed with AnalySIS software (Soft Imaging System).
Sample preparation for zymography
Tissue specimens of 20–40 mg were homogenized with an Ultra Turrax (Sigma) and lysed in lysis-buffer (1% Triton X-100, 20 mM Tris-HCl, 150 mM NaCl, 10 mM NaF in H2O). The protein concentration was determined (BCA Protein Assay 23227, Pierce Biotechnology, Rockford, IL) to allow loading of 60 μg protein/slot. Samples were stored at a temperature of −20°C.
Zymography
Myocardial extracts were loaded on 3% gelatin-containing (Sigma) gels (SDS-PAGE) with non-reducing loading buffer (125 mM Tris-HCl, 2% SDS, 10% glycine, 20 mM DTT, 0.01 bromophenol blue, pH 6.8). Gels were run at 20 mA/gel at 4°C with running buffer (25 mM Tris, 192 mM glycine, 0.1 SDS). Gels were then washed twice in 2.5% Triton X-100 for 30 min and incubated overnight at 37°C in substrate buffer (50 mM Tris-HCl, 5 mM CaCl2, pH 8). Finally, gels were washed with water and stained in Coomassie blue (0.25%). Subsequently, all gels were quantified by densitometry using the LumiImager Software (Roche, Mannheim, Germany) and results are expressed as artificial Boehringer Light Units (BLU). Experiments were performed in duplicate.
RNA isolation
For RNA isolation, samples were processed according to the RNeasy Mini Kit 74106 protocol (Qiagen, Hilden, Germany) including proteinase K and DNase digestion steps. RNA was then quantified by optical density using a Pharmacia Biotech Ultraspec 3000 (Pharmacia LKB, Freiburg, Germany) and then aliquoted and stored at −80°C until used.
Primers
The following primers were used: MMP-2: 5′-GCGACAAGAAGTATGGCTTC-3′, 3′-TGCCAAGGTCAATGTCAGGA-5′, 390 bp; MMP-9: 5′-CGCAGACATCGTCATC CAGT-3′, 3′-GGATTGGCCTTGGAAGATGA-5′, 406 bp; TIMP-1: 5′-CAATTCCGACCT CGTCATCA-3′, 3′-TCAGAGCCTTGGAGGAGCT-5′, 429 bp; TGF-β1: 5′-TGGCGATAC CTCAGCAACC-3′ and 3′-GATGCTGGGCCCTCTCCAG-5′. GAPDH: 5′-ATTCAAC GGCACAGTCAAGG-3′, 3′-CTTGTAGTAGGGACGTAGGT-5′.
RT-PCR and quantitative PCR
Equal amounts of RNA were transcribed using a 1st Strand cDNA Kit (Roche. The cDNA was stored at −20°C until used. Glassware was treated with 8.4 J UV-light. The reactions took place in 20 μl LightCycler capillaries using a Roche LightCycler (both Roche) with Lightcycler software version 3.5 (Idaho Technology, Salt Lake. City, UT). The LightCycler FastStart DNA Master SYBR Green I was used for the reaction mixture (Roche). The 40 amplification cycles consisted of an initial denaturation step at 95°C for 15 s followed by annealing at 60°C for 5 s and an elongation step at 72°C for 18 s (TIMP-1). The TIMP-1 cycle was completed by a 2 s step at 82°C. For TGF-β1 annealing took place at 62°C for 6 s followed by 15 s elongation and a final 5 s step at 87°C. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control. SYBR Green I fluorescence was detected after each cycle. Each sample was tested in duplicate and run twice.
Statistical analysis
Categorical data are given as absolute as well as corresponding relative frequencies (in %) and were compared in pairs between the four age groups using the χ 2-test. The distributions of the scores “NYHA” and “TR” were condensed by median and according to interquartile range. The nonparametric unpaired Wilcoxon test was computed for pair-wise comparison of these distributions between the four age groups. The obtained values of continuous variables are also summarised by arithmetic mean and corresponding standard deviation. Moreover, these two characteristic values were compared graphically between the four age groups. In addition, the unpaired t-test was conducted for pair-wise comparison of the calculated arithmetic means between the age groups.
A multifactorial covariance analysis was carried out to study the effects of the variables in Table 1 on collagen-content (in %). To reduce the number of factors incorporated in this analysis, factors with potentially relevant impact on fibrosis were selected using analysis of variance (ANOVA) for the age-groups, NYHA-, and TR-scores. Unpaired t-tests were used for all binary variables and simple linear regression analyses for continuous variables. Only factors yielding a P-value of <0.2 were considered to potentially influence fibrosis and were thus included in the multifactorial covariance analysis.
To evaluate a potential linear association between age (continuously measured) and collagen, a scatter plot was computed and further assessed using a simple linear regression analysis with the corresponding Pearson correlation coefficient.
A global significance level of α = 5% was chosen for all statistical test procedures. All statistical analyses were conducted in an explorative manner (Bender and Lange 2001), thus P-values ≤0.05 can be regarded as statistically significant results. SAS Statview for Windows Version 5.0 was used for statistical calculations.
Results
Atrial fibrosis
Based on Sirius red stainings (Fig. 2), age revealed a significant positive correlation (r = 0.45; P < 0.01) with morphometrically calculated fibrosis (Fig. 4a), and a significant negative correlation (r = −0.43; P < 0.01) with left ventricular function (i.e. EF). When results for fibrosis were analysed according to age in decades, we found a continuous increase from 10.1% ± 4.4% (≤50 years) to 16.7% ± 8.3% (>70 years) (Fig. 4b, Table 3). In parallel, the echocardiographically determined mean values of the ejection fraction (EF) decreased significantly with increasing age: patients younger than 50 years had an average EF of 53.5% ± 10.5%, which decreased to 42.8% ± 11.1% in the oldest group. Biatrial volume increased overall significantly with each additional decade of age. For all results and P-values, please refer to Table 1.
Graphical analysis of morphometrically determined fibrosis using Sirius-Red stainings. a Scatter plot and corresponding linear regression line displaying the degree of linear association between age and right atrial fibrosis (r = 0.45). b Graphical comparison of mean collagen content (and corresponding standard deviations) as percentage of total image area of the four age groups
Investigating possible associations between atrial fibrosis (i.e. Sirius red staining) and the factors listed in Table 1, a univariate analysis identified only age (measured continuously), coronary artery bypass graft (CABG), hypertension, EF, and angiotensin converting enzyme (ACE)-inhibition as potentially influencing factors (defined as a P-value <0.2). However, when the effect of these parameters on atrial fibrosis was analysed in a multifactorial covariance analysis, only age (P < 0.01) revealed an independent and statistically significant impact.
TGF-β and hypoxic signalling
Average TGF-β mRNA levels revealed a significant increase with age from 1.24 ± 0.02 in patients ≤50 years to 1.36 ± 0.03 in >70-year-old patients. Positive staining for HIF1α was significantly (P < 0.01) elevated in younger patients (compared to the oldest age group) at 30.7% ± 34.6% (≤50 years) and decreased with each decade of life to 0.8% ± 2.8% (>70 years). HIF1α exerts its effects, amongst others, through the VEGF-receptor KDR. Consequently, immunostaining for this receptor was examined, and, again, significantly (P < 0.05) elevated mean values were found at 72.1% ± 32.9% in patients under 50 years old. Subsequently, levels decreased and finally reached 33.6% ± 38.0% (>70 years). This age-related decrease in HIF1α and the VEGF-receptor KDR was followed by a simultaneous increase in PHD3 and a decrease in FIH, both of which may lessen the effects of HIF. FIH decreased from 59.2% ± 18.9% (≤50 years) to 29.2% ± 11.8% (>70 years) and PHD3 increased from 6.7% ± 4.9 (≤50 years) to 26.8% ± 11.8% (>70 years). Next we determined the endothelial marker CD34 (Fig. 3). Digital morphometric analysis found that MVD was significantly higher (P < 0.05) in patients ≤50 years of age with 15.8/mm2 ± 3.8/mm2 compared to the other age groups in which levels decreased to 9.6/mm2 ± 3.3/mm2 in patients >70 years (see Table 3).
MMP-2 and -9 and TIMP-1
The mRNA expression for MMP-9 and -2 and their zymographically determined pro-forms did not change significantly (data not shown). However, their zymographically active forms revealed a clear elevation with each additional decade of life for both MMP-9 and MMP-2. Average active MMP-9 levels increased from 4.3 BLU ± 0.8 BLU (≤50 years) to 52.5 BLU ± 11.4 BLU (>70 years). For average active MMP-2, values were 2.6 BLU ± 1.3 BLU (≤50 years) and subsequently increased step by step to 11.6 BLU ± 2.2 BLU (>70 years). These levels of activity for both enzymes were accompanied by a significantly decreased TIMP-1 mRNA level. In patients ≤50 years levels were 1.25 ± 0.05. From that level values decreased finally in increments to 1.11 ± 0.02 in patients >70 years of age (see Table 3).
Discussion
The major finding of this study is that the amount of atrial fibrosis increases with age. Pathophysiologically, our findings implicate—besides TGF-β and HIF1α—increased MMP activity and reduced TIMP expression as potential molecular mechanisms of atrial fibrogenesis. This is important because atrial fibrillation (AF) has been linked, for example, to changes in the atrial myocardium similar to those observed in this study (Gramley et al. 2007). This so called “atrial cardiomyopathy” may decrease the chance of successful cardioversion (Henry et al. 1976; Knackstedt et al. 2008) and explain why AF is particularly prevalent in elderly patients, promoting significant morbidity and mortality (Kannel et al. 1998). Understanding the underlying mechanisms of atrial fibrogenesis might thus allow new targets for early therapeutic intervention.
Age-related loss of ventricular cardiomyocytes has been well established (Olivetti et al. 1991). It has also been suggested that age plays a role during the development of cardiac fibrosis (Annoni et al. 1998; Gazoti Debessa et al. 2001; Robert et al. 1997). Studies in rats provide evidence for a positive relationship between age and ventricular fibrosis (Eghbali et al. 1989; Medugorac 1980). These studies point to continuous fibrogenesis by formation of new collagen fibres in the ventricles. The molecular basis of this process is unknown at present. Animal models suggest that there is a difference between age-related left- and right-ventricular fibrosis(Annoni et al. 1998; Rohrbach et al. 2005). Such differences may be related to chamber-specific haemodynamic conditions. Thus, findings in the ventricles cannot simply be transferred to the atria (Nakajima et al. 2000; O’Brien et al. 2000). Additionally, evidence from studies involving age-related human atrial fibrosis is scarce (Burkauskiene 2005; Nakai et al. 2007).
Another aspect of this work is its suggestion of potential molecular mechanisms of atrial fibrogenesis. We found TGF-β to be up-regulated with increasing age: our youngest group of patients had significantly lower average TGF-β mRNA levels than our oldest group of patients. Thus, in our study the amount of TGF-β may have contributed to age-related fibrogenesis. TGF-β is widely known for playing a pivotal role in cardiac fibrogenesis (Lijnen et al. 2000). A study with mutant TGF-β-deficient mice revealed that the loss of one TGF-β allele ameliorated age-associated myocardial fibrosis and improved LV compliance (Brooks and Conrad 2000). This supports the importance of TGF-β during age-related fibrosis. Interestingly, another study performed with Sprague-Dawley rats investigated fibrosis and TGF-β levels at various time points between 2 and 19 months of age and found that TGF-β was not significantly altered, but collagens (Type I and III) were differentially changed in RV and LV (Annoni et al. 1998). These differences may reflect (1) potential differences between species, and/or (2) an observational time span (maximal rat age of 19 months) not comparable with the age span in this study.
A deficient oxygen supply may result in cardiac remodelling, fibrogenesis and myocardial dysfunction (Pelouch et al. 1997). Such a dysfunction may be ventricular—but may also involve the atria (atrial cardiomyopathy). Increased atrial volume is one sign of atrial dysfunction (Schotten et al. 2003). Atrial function can be assessed using the echocardiographic strain rate. Increased atrial volume (and age!) is associated with a reduction of atrial strain rate (Inaba et al. 2005; Kokubu et al. 2007) and an increase of myocardial stress and stretch (Epstein and Davis 2003; Schotten et al. 2003). Left ventricular myocardial stretch has been shown to induce TGF-β, which in turn may up-regulate pro-angiogenic signals (Li et al. 1997). We found average values of HIF1α and the VEGF-receptor KDR to decrease with increasing age. This points to a reduction in age-related hypoxic signalling. A similar decrease in HIF1α with age has been reported for brain, liver, and kidney tissue (Frenkel-Denkberg et al. 1999). Another recent study found HIF1α to decrease and PHD3 to increase with increasing age; the latter showed a strong inducibility by ischemia/hypoxia (Rohrbach et al. 2005). In our study, these findings were complemented by a positive association between age and FIH. With increasing age, adaptation towards hypoxia, including angiogenesis, has been shown to be reduced (Cataldi et al. 2004; Rivard et al. 1999, 2000). In the present study, we found such an age-dependent reduction in atrial MVD. In conclusion, our data point to a negative association between age and hypoxic signalling, which may have led to the observed lower MVD level and, thus, a potentially reduced adaptation to hypoxia in old age.
As an important part of the interstitial matrix, collagen is a central component of myocardial tissue. This matrix surrounds and thus supports cardiomyocytes and their alignment during the cardiac cycle (Borg and Caulfield 1981; Robinson et al. 1983). Therefore, collagen (and fibrosis) plays an important role in the elastic and pathological properties of the myocardium. The present study is the first to prove a positive correlation between age and the gelatinases MMP-9 and -2, and a corresponding negative association between age and the mRNA of their common tissue inhibitor TIMP-1. Recently, we found increasing amounts of fibrosis in AF to be associated with rising levels of MMPs and falling levels of TIMPs (Gramley et al. 2007). A similar pattern with atrial up-regulation of MMPs and a concomitant down-regulation of TIMPs has also been found in patients with heart failure (Xu et al. 2004). Another study suggests a link between atrial fibrogenesis, the up-regulation of MMPs and haemodynamic overload in patients with heart failure (Boixel et al. 2003). The present study, however, can demonstrate that the coexistence of heart failure—the majority of our patients had either preserved or only moderately reduced left ventricular functions—appears not to be mandatory. Despite significant variations in EF among the age groups (EF between 53.5% ± 10.5% and 42.8% ± 11.1%) this did not statistically influence atrial fibrosis as proven by multifactorial covariance analysis. Rather, it seems that age-related changes with subsequently increased atrial volumes (possibly induced by atrial pressure overload) are sufficient to cause atrial remodelling—very much as in vascular walls that adapt to increased systolic blood pressure by undergoing age-related structural changes. This concept is further supported by comparable changes in MMPs and TIMPs in a canine model of pacing-induced lone atrial cardiomyopathy (Hoit et al. 2002). Thus, an increase in atrial fibrosis may represent a response to increased haemodynamic loading (White et al. 1982).
Limitations
The present study cannot answer the question as to whether age-dependent fibrosis and the observed molecular abnormalities are merely associated with one another or whether there is an underlying causal relationship.
Pre-existing medication with ACE-inhibitors and AT1-receptor blockers in the participants in our study may have affected the degree of atrial fibrosis (Goette et al. 2000; Vermes et al. 2003) and the expression of components of the MMP system. The rationale for continuing ACE-I in the subjects studied was to not withhold a potential benefit from the patient. However, the fact that the average percentage of the recommended maximum dose of these drugs was only 40.0% ± 15.3% indicates that this dose may have been insufficient to block all antifibrotic effects of AT II (statistical multifactorial covariance analysis supports this lack of influence of ACE-inhibition on fibrogenesis). In addition, similar findings of MMP expression/activity associated with atrial fibrosis in experimental settings without ACE-inhibition (Boixel et al. 2003; Hoit et al. 2002) may be taken as further evidence of the role of this system in atrial fibrogenesis.
Since, by protocol, left atrial tissue was not obtained, possible differences between right and left atrial remodelling could not be investigated.
Finally, since all of the patients investigated suffered from organic heart disease, it is impossible to say to what degree and in what way this part of their past medical history may have contributed to structural atrial remodelling.
Conclusion
With increasing age, atrial tissue of patients in sinus rhythm develops significantly more fibrosis. An increase in fibrotic and a decrease in hypoxic signalling and MVD coupled with a differential expression of MMPs and TIMP-1 favouring fibrosis may have contributed to age-related atrial fibrogenesis (Fig. 1). Finally, age was the only factor statistically proven to have influenced atrial fibrosis.
Abbreviations
- ACE:
-
Angiotensin converting enzyme
- CABG:
-
Coronary artery bypass graft
- EF:
-
Ejection fraction
- FIH:
-
Factor inhibiting HIF
- HIF:
-
Hypoxia induced factor
- HRE:
-
Hypoxia response elements
- IHC:
-
Immunohistochemistry
- KDR:
-
Kinase insert-domain containing receptor (= VEGF-receptor)
- LA:
-
Left atrium
- LV:
-
Left ventricle
- MMP:
-
Matrix-metalloproteinases
- MVD:
-
Microvessel density
- PHD3:
-
Prolyl hydroxylases 3
- RA:
-
Right atrium
- RAA:
-
Right atrial appendage
- RV:
-
Right ventricle
- TGF-β:
-
Transforming growth factor-β
- TIMP:
-
Tissue inhibitors of metalloproteinases
- VEGF:
-
Vascular endothelial growth factor
References
Anne W, Willems R, Roskams T et al (2005) Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res 67(4):655–666 doi:10.1016/j.cardiores.2005.04.016
Annoni G, Luvara G, Arosio B et al (1998) Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mech Ageing Dev 101(1–2):57–72 doi:10.1016/S0047-6374(97)00165-6
Bender R, Lange S (2001) Adjusting for multiple testing—when and how? J Clin Epidemiol 54(4):343–349 doi:10.1016/S0895-4356(00)00314-0
Boixel C, Fontaine V, Rucker-Martin C et al (2003) Fibrosis of the left atria during progression of heart failure is associated with increased matrix metalloproteinases in the rat. J Am Coll Cardiol 42(2):336–344 doi:10.1016/S0735-1097(03)00578-3
Borg TK, Caulfield JB (1981) The collagen matrix of the heart. Fed Proc 40(7):2037–2041
Brilla CG (2000a) Aldosterone and myocardial fibrosis in heart failure. Herz 25(3):299–306 doi:10.1007/s000590050024
Brilla CG (2000b) Regression of myocardial fibrosis in hypertensive heart disease: diverse effects of various antihypertensive drugs. Cardiovasc Res 46(2):324–331 doi:10.1016/S0008-6363(99)00432-0
Brooks WW, Conrad CH (2000) Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol 32(2):187–195 doi:10.1006/jmcc.1999.1065
Burkauskiene A (2005) Age-related changes in the structure of myocardial collagen network of auricle of the right atrium in healthy persons and ischemic heart disease patients. Medicina (Kaunas) 41(2):145–154
Cataldi A, Bianchi G, Rapino C et al (2004) Molecular and morphological modifications occurring in rat heart exposed to intermittent hypoxia: role for protein kinase C alpha. Exp Gerontol 39(3):395–405 doi:10.1016/j.exger.2003.11.010
Cioffi CL, Liu XQ, Kosinski PA et al (2003) Differential regulation of HIF-1 alpha prolyl-4-hydroxylase genes by hypoxia in human cardiovascular cells. Biochem Biophys Res Commun 303(3):947–953 doi:10.1016/S0006-291X(03)00453-4
De Boer RA, Pinto YM, Van Veldhuisen DJ (2003) The imbalance between oxygen demand and supply as a potential mechanism in the pathophysiology of heart failure: the role of microvascular growth and abnormalities. Microcirculation 10(2):113–126 doi:10.1080/713773607
Eghbali M, Robinson TF, Seifter S et al (1989) Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res 23(8):723–729 doi:10.1093/cvr/23.8.723
Epstein ND, Davis JS (2003) Sensing stretch is fundamental. Cell 112(2):147–150 doi:10.1016/S0092-8674(03)00037-0
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9(6):669–676 doi:10.1038/nm0603-669
Frenkel-Denkberg G, Gershon D, Levy AP (1999) The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett 462(3):341–344 doi:10.1016/S0014-5793(99)01552-5
Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R (2001) Age related changes of the collagen network of the human heart. Mech Ageing Dev 122(10):1049–1058 doi:10.1016/S0047-6374(01)00238-X
Goette A, Arndt M, Rocken C et al (2000) Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 101(23):2678–2681
Goette A, Juenemann G, Peters B et al (2002) Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res 54(2):390–396 doi:10.1016/S0008-6363(02)00251-1
Gramley F, Lorenzen J, Plisiene J et al (2007) Decreased plasminogen activator inhibitor and tissue metalloproteinase inhibitor expression may promote increased metalloproteinase activity with increasing duration of human atrial fibrillation. J Cardiovasc Electrophysiol 18(10):1076–1082 doi:10.1111/j.1540-8167.2007.00906.x
Hao J, Ju H, Zhao S et al (1999) Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol 31(3):667–678 doi:10.1006/jmcc.1998.0902
Harris AL (2002) Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer 2(1):38–47 doi:10.1038/nrc704
Henry WL, Morganroth J, Pearlman AS et al (1976) Relation between echocardiographically determined left atrial size and atrial fibrillation. Circulation 53(2):273–279
Ho K, Pinsky J, Kannel W et al (1993) The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 22:6A–13A
Hoit BD, Takeishi Y, Cox MJ et al (2002) Remodeling of the left atrium in pacing-induced atrial cardiomyopathy. Mol Cell Biochem 238(1–2):145–150 doi:10.1023/A:1019988024077
Inaba Y, Yuda S, Kobayashi N et al (2005) Strain rate imaging for noninvasive functional quantification of the left atrium: comparative studies in controls and patients with atrial fibrillation. J Am Soc Echocardiogr 18(7):729–736 doi:10.1016/j.echo.2004.12.005
Kannel W, Abbot R, Savage D et al (1982) Epidemiological features of atrial fibrillation. The Framingham study. N Engl J Med 306:1018–1022
Kannel WB, Wolf PA, Benjamin EJ et al (1998) Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 82(8A):2N–9N doi:10.1016/S0002-9149(98)00583-9
Khan R, Sheppard R (2006) Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 118(1):10–24 doi:10.1111/j.1365-2567.2006.02336.x
Knackstedt C, Gramley F, Schimpf T et al (2008) Association of echocardiographic atrial size and atrial fibrosis in a sequential model of congestive heart failure and atrial fibrillation. Cardiovasc Pathol 17(5):318–324
Kokubu N, Yuda S, Tsuchihashi K et al (2007) Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin-angiotensin system inhibition on left atrial function. Hypertens Res 30(1):13–21 doi:10.1291/hypres.30.13
Li J, Hampton T, Morgan JP et al (1997) Stretch-induced VEGF expression in the heart. J Clin Invest 100(1):18–24 doi:10.1172/JCI119510
Li X, Ma C, Dong J et al (2008) The fibrosis and atrial fibrillation: is the transforming growth factor-beta(1) a candidate etiology of atrial fibrillation. Med Hypotheses 70(2):317–319 doi:10.1016/j.mehy.2007.04.046
Lijnen PJ, Petrov VV, Fagard RH (2000) Induction of cardiac fibrosis by transforming growth factor-beta(1). Mol Genet Metab 71(1–2):418–435 doi:10.1006/mgme.2000.3032
Medugorac I (1980) Collagen content in different areas of normal and hypertrophied rat myocardium. Cardiovasc Res 14(9):551–554 doi:10.1093/cvr/14.9.551
Mukherjee R, Herron AR, Lowry AS et al (2006) Selective induction of matrix metalloproteinases and tissue inhibitor of metalloproteinases in atrial and ventricular myocardium in patients with atrial fibrillation. Am J Cardiol 97(4):532–537 doi:10.1016/j.amjcard.2005.08.073
Nakai T, Chandy J, Nakai K et al (2007) Histologic assessment of right atrial appendage myocardium in patients with atrial fibrillation after coronary artery bypass graft surgery. Cardiology 108(2):90–96 doi:10.1159/000095936
Nakajima H, Nakajima HO, Salcher O et al (2000) Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res 86(5):571–579
O’Brien DW, Fu Y, Parker HR et al (2000) Differential morphometric and ultrastructural remodelling in the left atrium and left ventricle in rapid ventricular pacing-induced heart failure. Can J Cardiol 16(11):1411–1419
Ohtani K, Yutani C, Nagata S et al (1995) High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J Am Coll Cardiol 25(5):1162–1169 doi:10.1016/0735-1097(94)00529-Y
Olivetti G, Melissari M, Capasso JM et al (1991) Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res 68(6):1560–1568
Pelouch V, Kolar F, Ost’adal B et al (1997) Regression of chronic hypoxia-induced pulmonary hypertension, right ventricular hypertrophy, and fibrosis: effect of enalapril. Cardiovasc Drugs Ther 11(2):177–185 doi:10.1023/A:1007788915732
Rivard A, Fabre JE, Silver M et al (1999) Age-dependent impairment of angiogenesis. Circulation 99(1):111–120
Rivard A, Berthou-Soulie L, Principe N et al (2000) Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem 275(38):29643–29647 doi:10.1074/jbc.M001029200
Robert V, Besse S, Sabri A et al (1997) Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest 76(5):729–738
Robinson TF, Cohen-Gould L, Factor SM (1983) Skeletal framework of mammalian heart muscle. Arrangement of inter- and pericellular connective tissue structures. Lab Invest 49(4):482–498
Rohrbach S, Simm A, Pregla R et al (2005) Age-dependent increase of prolyl-4-hydroxylase domain (PHD) 3 expression in human and mouse heart. Biogerontology 6(3):165–171 doi:10.1007/s10522-005-7950-9
Sanfilippo AJ, Abascal VM, Sheehan M et al (1990) Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 82(3):792–797
Schotten U, Neuberger HR, Allessie MA (2003) The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol 82(1–3):151–162 doi:10.1016/S0079-6107(03)00012-9
Seeland U, Haeuseler C, Hinrichs R et al (2002) Myocardial fibrosis in transforming growth factor-beta(1) (TGF-beta(1)) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest 32(5):295–303 doi:10.1046/j.1365-2362.2002.00985.x
Sivasubramanian N, Coker ML, Kurrelmeyer KM et al (2001) Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104(7):826–831 doi:10.1161/hc3401.093154
Staessen JA, Wang JG, Thijs L et al (1999) Overview of the outcome trials in older patients with isolated systolic hypertension. J Hum Hypertens 13(12):859–863 doi:10.1038/sj.jhh.1000899
Tanaka T, Kato H, Kojima I et al (2006) Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci 61(8):795–805
Thijssen VL, van der Velden HM, van Ankeren EP et al (2002) Analysis of altered gene expression during sustained atrial fibrillation in the goat. Cardiovasc Res 54(2):427–437 doi:10.1016/S0008-6363(02)00260-2
Vermes E, Tardif JC, Bourassa MG et al (2003) Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation 107(23):2926–2931 doi:10.1161/01.CIR.0000072793.81076.D4
Wang B, Hao J, Jones SC et al (2002) Decreased Smad 7 expression contributes to cardiac fibrosis in the infarcted rat heart. Am J Physiol Heart Circ Physiol 282(5):H1685–H1696
White CW, Kerber RE, Weiss HR et al (1982) The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ Res 51(2):205–215
Xu J, Cui G, Esmailian F et al (2004) Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation 109(3):363–368 doi:10.1161/01.CIR.0000109495.02213.52
Author information
Authors and Affiliations
Corresponding author
Additional information
No author has any conflicts of interest to disclose.
About this article
Cite this article
Gramley, F., Lorenzen, J., Knackstedt, C. et al. Age-related atrial fibrosis. AGE 31, 27–38 (2009). https://doi.org/10.1007/s11357-008-9077-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-008-9077-9