Abstract
Shorea is the largest and most important genus of the Dipterocarpaceae. The genetic diversity and structure of nine Shorea species from two different locations, namely Nanjak Makmur in Sumatra and Sumalindo in Borneo, were evaluated using amplified fragment length polymorphism (AFLP) markers. A total of 274 trees were investigated at 85 polymorphic AFLP loci. Levels of genetic diversity of these species ranged from \(\overline {H_{\text{e}} } \) = 0.100 for S. acuminata to \(\overline {H_{\text{e}} } \) = 0.165 for S. blumutensis. The population of rare species S. blumutensis possessed the highest genetic diversity suggesting that geographically restricted species can have levels of genetic variation comparable to closely related widespread common congeners. Analyses of molecular variance revealed that the genetic variation was mainly found among species in both locations (57.7% in Sumatra; 56.3% in Borneo). The unweighted pairgroup method using arithmetic averages dendrogram of all samples revealed an almost complete separation of species. Thus, AFLP markers proved appropriate for phylogenetic studies of Shorea species. Specific markers have been detected showing high-frequency differences among species and between regions within species. Sequence information of these markers can be used to develop specific polymerase chain reaction markers for wood identification. The possibility of interspecific hybridization was discussed.
Similar content being viewed by others
Introduction
Shorea Roxb. is the dominant emergent tree genus in tropical Asia (Ashton 1982). It is the largest genus of the Dipterocarpaceae, the most important tree family in tropical Southeast Asia both from an ecological and an economic perspective. It encompasses about 200 species, of which 163 are distributed in Malesia, mostly in Indonesia, in particular on Sumatra and Borneo (Kalimantan). The timber is highly valued and used for construction (shipbuilding, bridges, piers), decking, and outdoor furniture (Ashton 1982). The distribution area and the abundance of many Shorea species in Indonesia are shrinking due to the changes of land use systems from forests to other uses including agriculture and cultivation of oil palms, and due to the exploitation of the valuable timber. The genetic consequences of forest decline on dipterocarps are largely unknown. Patterns of genetic variation within and among species have rarely been studied in primary and secondary dipterocarp forests in Indonesia, although an understanding of the spatial distribution of genetic diversity is crucial for the development of strategies for an efficient conservation and sustainable use of genetic resources.
The development of DNA-based molecular marker techniques has advanced the studies of genetic diversity considerably over the last decade, which allows fingerprinting of plant genomes and reveals direct and reliable polymorphisms at the DNA level (e.g., De Verno and Mosseler 1997; Rogers 2002). We chose amplified fragment length polymorphisms (AFLPs; Vos et al. 1995) for this study. Due to the high multiplex ratio (a large number of polymorphic loci generated in a single experiment; Rafalski et al. 1996) and reproducibility (Jones et al. 1997), AFLP is an efficient marker technique for fingerprinting and assessing genetic polymorphisms (Garcia et al. 2004; Xiao et al. 2006; Bouajila et al. 2007; Masum Akond et al. 2008). This marker technique has often been applied to study of genetic variation in forest tree species (Gailing and von Wuehlisch 2004; Castillo-Càrdenas et al. 2005; Lara-Gomez et al. 2005; Tang et al. 2008). The application of AFLPs as simple, universal markers for assessment of genetic variation of tropical forest trees has recently been emphasized by Kremer et al. (2005), but only few reports based on the AFLP marker technique are available for Shorea (Cao et al. 2006a, b) and any other dipterocarps (Luu 2005).
The objectives of the present study are: (1) to evaluate genetic variation within and differentiation among nine Shorea species from two different locations, namely Nanjak Makmur on Sumatra and Sumalindo on Borneo using AFLP markers; (2) to test if AFLPs are suitable markers to distinguish among species, between regions within species, and to dissect phylogenetic relationships among species within the genus Shorea; and (3) to test whether widespread common dipterocarp species possess more genetic variation within populations than rare species or species with scattered distribution. Furthermore, we want to detect AFLP markers with pronounced frequency differences among species, and between regions within species, which contribute to development of molecular genetic tools for the identification of wood from different species and origins.
Materials and methods
Plant material
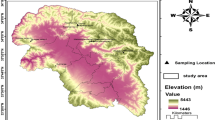
The leaves of adult trees and saplings were collected from 11 natural populations of nine Shorea species distributed in two different locations (Nanjak Makmur in Sumatra and Sumalindo in Borneo) in Indonesia. The six populations from Nanjak Makmur Sumatra are located at an elevation of approximately 100 m, 10°22′ S and 101°40′ E, including the two populations Spar_NS and Slep_NS previously studied using AFLPs (Cao et al. 2006a). The five populations from Sumalindo Borneo are located at 200 m, 00°55′–00°56′ N and 115°18′–116°36′ E. Information on the taxonomic status of the nine Shorea species and species distribution on islands Sumatra and Borneo, location, and sample size of populations is shown in Table 1 and Fig. 1. According to Ashton (1982) and Newman et al. (1996a, b), distributions of Shorea species studied are somewhat different from each other. S. parvifolia is the most common dipterocarp species and distributed in Sumatra and Borneo, followed by S. leprosula, S. palembanica and S. johorensis. S. leprosula is very common in Sumatra, but much less common in northwestern and eastern Borneo. S. palembanica is common on river banks and freshwater swamps and rarely occurs on low moist hillsides in Sumatra and Borneo. S. johorensis is found in eastern Sumatra and commonly in eastern Borneo. S. acuminata has a narrow distribution, because it is commonly found only in Sumatra. Over a wide altitudinal range, S. dasyphylla is quite widespread throughout Sumatra and Borneo, but has a scattered distribution compared to other Shorea species. S. blumutensis is confined only to northeastern Sumatra, with rare occurences in lowland dipterocarp forests. Shorea macroptera has four subspecies with different geographical distributions in Sumatra (ssp. macroptera) and Borneo (the other three subspecies), only ssp. macroptera is subject to the AFLP study. S. platyclados is widespread in mountainous districts usually between 700 and 1,300 m, and not as common as S. parvifolia, S. leprosula, and S. palembanica.
Geographic distribution of 11 populations of nine Shorea species studied. 1, S. parvifolia; 2, S. acuminata; 3, S. dasyphylla; 4, S. blumutensis; 5, S. leprosula; 6, S. macroptera; 7, S. parvifolia; 8, S. leprosula; 9, S. palembanica; 10, S. platyclados; 11, S. johorensis; NS Nanjak Makmur Sumatra, SLB Sumalindo Borneo
The collection site for each species within regions had an area of 100–300 ha. A minimum distance of 30 m was kept between sample trees to avoid excessive sampling of related plants. Populations have a minimum sample size of 20 trees (except for S. leprosula in Nanjak Makmur, n = 16). The estimated density for the nine species is 0.15 to 3 trees per hectare. Species identification was done on the basis of leaf morphological characters such as leaf length, petiole length, leaf width, distance from petiole to the widest part of the leaf, number of venation, number of lobes, dometiana length, and leaf shape.
DNA extraction
Total genomic DNA was extracted from a small slice (ca. 2 cm2) of silica gel-dried leaf tissue following the DNeasy 96 Plant Kit protocol of the manufacturer (Qiagen, Hilden, Germany). Five-microliter DNA was separated electrophoretically on a 0.8% agarose gel at 100 V in TAE buffer, visualized by staining with ethidium bromide, and photographed in ultraviolet light to check DNA quantity and quality. Extracted DNA was stored at −20°C.
AFLP assays
The AFLP fingerprinting technique of Vos et al. (1995) was employed with slight modifications. Total genomic DNA of each sample was digested with the two restriction endonucleases EcoRI and MseI. The EcoRI-adaptor and the MseI-adaptor were ligated to the ends of the restriction fragments. The restriction–ligation reactions were performed overnight (14–16 h) at room temperature to generate template DNA for polymerase chain reaction (PCR) amplification. The preselective amplifications were conducted using primer pair E01/M03 with selective nucleotides A and G, respectively. The selective amplifications were achieved using primer pair E35/M63 having selective nucleotides ACA and GAA, respectively. Primer E35 was labeled with fluorescent dye 6-FAM. All PCR reactions were conducted in the Peltier Thermal Cycler (PTC-200 version 4.0, MJ Research). The amplified restriction products were separated electrophoretically on the ABI Genetic Analyser 3100 together with the internal size standard GeneScan 500 ROX (fluorescent dye ROX) from Applied Biosystems. The size of the AFLP fragments was detected with the software packages GeneScan 3.7 and Genotyper 3.7 (Applied Biosystems).
Data analysis of AFLP markers
Each AFLP band was assumed to correspond to a dominant allele at a single locus. Only unambiguous bands of total AFLP fingerprint patterns were manually selected and scored as present (1) or absent (0) in each sample. Binary character matrices were compiled for further analysis. The exact test for linkage disequilibrium was conducted for all marker pairs in each species using the program ARLEQUIN version 3.01 (Excoffier et al. 2006).
POPGENE version 1.31 software (Yeh et al. 1999) was used to calculate the parameters of genetic variation under the assumption of Hardy–Weinberg equilibrium, including the percentage of polymorphic loci (PPL; Nei 1973), effective number of alleles per locus (n e; Hartl and Clark 1989), gene diversity (h = expected heterozygosity H e; Nei 1973), Shannon’s information index for phenotypic diversity quantifying the degree of AFLP polymorphism within populations (I = −Σp i log2 p i , where p i is the frequency of the presence or absence of a AFLP band; Lewontin 1972), total genetic diversity (H t), genetic diversity within populations (H s), genetic diversity among populations (D st), and the relative magnitude of genetic differentiation among populations (G st = D st/H t; Nei 1987). Allelic frequencies were calculated based on the square root of the frequency of the null (recessive) allele. Nei’s (1978) unbiased measures of genetic identity and genetic distance were calculated for all pairwise combinations of populations. An unweighted pairgroup method using arithmetic averages (UPGMA) dendrogram was constructed using the program NTSYS-pc version 2.0 (Rohlf 1998) based on Nei’s (1978) genetic distances. Hierarchical analyses of molecular variance (AMOVA; Excoffier et al. 1992) based on the pairwise squared Euclidean distances among molecular phenotypes (presence or absence of bands) were conducted using ARLEQUIN version 3.01 (Excoffier et al. 2006) to further quantify the amount of genetic variation residing at two levels (i.e., among and within populations; among species and within species on each island). The same program was used to generate the matrix of pairwise F st values, which indicate the genetic differentiation between populations, and are analogous to G st if a locus consists of two alleles as applicable in dominant marker analyses (e.g., RAPD; Nybom and Bartish 2000). The significance levels for AMOVA were evaluated using a permutation approach (1,023 replications).
Another UPGMA clustering indicating the patterns of variation within and among populations was performed based on similarity values using the software NTSYS-pc version 2.0 (Rohlf 1998). The estimates of genetic similarity were calculated from all possible pairwise combinations of individuals according to Dice index (1945): S ij = 2a/(2a + b + c), where S ij is the similarity between two individuals i and j, a is the number of bands present in both i and j, b is the number of bands present in i and absent in j, and c is the number of bands present in j and absent in i. The goodness of fit of the clustering to the genetic distance matrix, on which it was based, was calculated using the Mantel z statistic (Mantel 1967; Rohlf 1998).
Results
Test for linkage disequilibrium
The linkage disequilibrium for all marker pairs was tested in each species. The percentage of loci in significant linkage disequilibrium (p < 0.01) ranged from 3.6% for S. macroptera to 14.3% for S. platyclados, suggesting independence of most AFLP markers.
Genetic diversity
A total of 85 unequivocally recognizable polymorphic AFLP fragments were scored, ranging in size from 76 to 486 bp. Table 2 shows that the genetic diversity varied among species. At the single species level, the rare species S. blumutensis and the scattered species S. dasyphylla possessed the highest level of diversity with the values of n e = 1.266, H e = 0.165, I = 0.257, and n e = 1.273, H e = 0.164, I = 0.251, respectively. The relatively widespread species S. acuminata harbored the lowest diversity values of n e = 1.159, H e = 0.100, I = 0.162. There was no positive association between the level of genetic variation and the extent of distribution of a species.
Genetic structure
The results of AMOVAs (Table 3) showed highly significant (p < 0.001) genetic differentiation among populations (mostly among species). The main portion of the total variation resided among species. AMOVAs at two hierarchical levels indicated remarkable values of among-species variation in Nanjak Makmur Sumatra (57.7%) and in Sumalindo Borneo (56.3%). Likewise, genetic differentiation among species (G st) calculated using the POPGENE software (Yeh et al. 1999) was high in Nanjak Makmur Sumatra (G st = 0.52) and in Sumalindo Borneo (G st = 0.46). The pairwise F st values (Table 4) generated from AMOVA indicated that all populations were significantly differentiated (p < 0.05) with a range from 0.297 (Sdas_NS/Spar_SLB) to 0.711 (Sblu_NS/Slep_SLB). The F st values between S. dasyphylla and S. parvifolia showed the lowest level (F st = 0.297 for Sdas_NS/Spar_SLB and F st = 0.312 for Sdas_NS/Spar_NS) followed by F st values of 0.336 for the population pair of S. parvifolia (Spar_NS/Spar_SLB) and 0.404 for the S. leprosula pair (Slep_NS and Slep_SLB).
Genetic distances and cluster analyses
Table 5 shows Nei’s (1978) pairwise genetic distances between populations. The largest distance (0.389) was found between S. blumutensis and S. leprosula in Nanjak Makmur Sumatra (Sblu_NS/Slep_NS), and the smallest (0.067) between the two populations of S. leprosula from the two different regions (Slep_NS/Slep_SLB). Additionally, small genetic distances were also found between the two populations of S. parvifolia (0.087 for Spar_NS/Spar_SLB), and between the species S. dasyphylla and S. parvifolia (0.094 for Sdas_NS/Spar_NS, 0.077 for Sdas_NS/Spar_SLB).
An UPGMA dendrogram (Fig. 2) displayed the genetic relationships among all the 11 populations of nine species investigated based on the matrix of Nei’s (1978) genetic distances (Table 5). In most cases, the species were clearly separated from each other, but one population of S. parvifolia (Spar_SLB) and S. dasyphylla (Sdas_NS) formed one cluster as sister to the other population of S. parvifolia (Spar_NS). The clustering of these Shorea species was in accordance with the division of wood varieties. Nevertheless, the dendrogram only partially reflected the taxonomic subdivision among sections. S. blumutensis (Sblu_NS, sect. Richetioides, Yellow Meranti) was divergent from all the other species of the wood variety Red Meranti. Within the Red Meranti group, S. acuminata (Sacu_NS, sect. Mutica subsect. Mutica) branched first followed by the two populations of S. leprosula (Slep_SLB, Slep_NS, sect. Mutica subsect. Mutica) and a larger cluster. The latter cluster consisted of two sister groups, one containing S. parvifolia and S. dasyphylla (Spar_NS, Spar_SLB and Sdas_NS, sect. Mutica subsect. Mutica), and the other containing S. palembanica, S. platyclados, S. johorensis (Spal_SLB, Splat_SLB, and Sjoh_SLB, sect. Brachypterae subsect. Brachypterae), and S. macroptera (Smac_NS, sect. Mutica subsect. Auriculatae).
Genetic relationships among all the individuals of 11 populations of the nine species were revealed further by another UPGMA analysis based on the Dice (1945) similarity index (Fig. 3a, b). Individuals of the same population and species were grouped together in the same cluster except for three individuals (S. platyclados: 2220_pc_SLB and 2217_pc_SLB; S. dasyphylla: 1221_d_NS; see arrows in Fig. 3a, b), for which the genetic assignment was not consistent with the morphological diagnosis. Nine species can be clearly identified in nine clusters. Individuals of S. parvifolia, S. dasyphylla, and S. leprosula were grouped in one large cluster (Fig. 3a). S. macroptera, S. johorensis, and S. platyclados formed another large cluster. S. palembanica, S. acuminata, and S. blumutensis clustered to the base of the tree in separate clusters. Samples of S. blumutensis were separated far from the individuals of the other species (Fig. 3b). Generally, the topology of Fig. 3 is similar to that of Fig. 2. All the individuals were clearly divided into two wood varieties, Red Meranti and Yellow Meranti. Within Red Meranti, the separation of sections (Brachypterae and Mutica) was not clear. S. macroptera from section Mutica (subsection Auriculatae) grouped together with species of section Brachypterae. Different from Fig. 2, S. parvifolia populations from Sumatra and Borneo grouped together in one cluster which is sister to S. dasyphylla. The cophenetic correlation showed a very good fit of the cluster analysis to the matrix of genetic distances (r = 0.88).
Distribution of AFLP markers
The genetic diversity revealed at AFLPs is attributed to the differences in distribution patterns of markers in individuals. Some AFLP markers showed remarkable frequency differences not only among species, but also between regions within the species S. leprosula and S. parvifolia (Fig. 4). Markers 51 (a) and 60 (b) are private to S. platyclados and S. johorensis, respectively, with high frequencies (>0.8). Marker 30 (c), 37 (d), and 54 (e) have high frequencies (>0.9) in S. palembanica, S. blumutensis, and S. acuminata, respectively, and low frequencies (<0.2) in the other species (see also Fig. 3b). Marker 61 (f) shows a high frequency (1.0) in S. macroptera and low frequencies (<0.1) in most other species. In Sumalindo Borneo, marker 32 (g) shows a high frequency (>0.9) only in S. johorensis and low frequencies (<0.2) in the other species. The same marker 32 (g) is present with high frequencies (>0.9) in S. blumutensis and S. leprosula in Nanjak Makmur Sumatra. This is an indication that fragments of the same size may be not homologous. Markers 32 (g) and marker 45 (h) are present in Nanjak Makmur Sumatra and Sumalindo Borneo, respectively, with high frequencies (>0.9) for S. leprosula and absent in the other region. Markers 39 (i) and 73 (j) differentiate between Nanjak Makmur Sumatra and Sumalindo Borneo for S. parvifolia. In Sumalindo Borneo, marker 39 (i) has a high frequency (>0.8) in S. platyclados and a low frequency (<0.2) in the other species. The homology of equal-sized fragments in different species has to be tested (see “Discussion”).
Discussion
Genetic diversity within species
Nei’s gene diversity (H e) and Shannon’s information index (that does not rely on the estimation of allele frequencies) showed identical tendencies (Table 2). Since most studies applied H e as diversity estimate (Cao et al. 2006a), the present study focused on this measure to compare genetic diversity among species. We are aware that the relatively small number of 85 fragments may result in biases in the estimation of genetic diversity (Szmidt et al. 1996; Isabel et al. 1999). Estimates of genetic diversity are dependent on the frequency of null homozygotes and the fixation index F of populations. Multilocus estimates of gene diversity from a higher number of markers are more robust to variations in the fixation index F of populations (Kremer et al. 2005). Since Shorea species are predominantly outcrossing (Ashton 1969; Chan 1981; Sakai et al. 1999; Lee et al. 2000), the assumption of Hardy–Weinberg equilibrium can be justified. The absence of linkage disequilibrium for most marker pairs in the present study and in earlier study on S. leprosula and S. parvifolia using the same AFLP primer combination suggests that genome-wide variation patterns can be assessed even by the comparatively limited number of fragments (Cao et al. 2006a).
The breeding system in plants is one factor that affects gene flow, and hence influences the genetic variation within and among populations (Loveless and Hamrick 1984). Outcrossing is predominant in many tropical tree species (Nason and Hamrick 1996). Shorea species are predominantly outcrossing and strongly self-incompatible (Chan 1981; Sakai et al. 1999; Lee et al. 2000; Nagamitsu et al. 2001; Obayashi et al. 2002). Outcrossing plants in general exhibit higher levels of genetic diversity within populations than selfing plants (Hamrick and Godt 1996).
Compared to other tree species (including dipterocarps) studied using AFLPs (Castillo-Càrdenas et al. 2005; Lara-Gomez et al. 2005; Luu 2005; Tang et al. 2008), the nine Shorea species investigated here showed moderate levels of genetic diversity (H e) ranging from 0.100 (S. acuminata) to 0.165 (S. blumutensis). The differences of genetic diversity estimates among species are small. These levels of genetic diversity can be attributed to the population evolutionary history of Shorea species like bottleneck effects and genetic drift at the last glacial maximum when tropical rain forests were restricted to comparatively small refugia on Borneo and Sumatra (Gathorne-Hardy et al. 2002). However, no evidence for strong bottleneck effects was found for any of the investigated species.
Comprehensive studies on more than 141 plant species revealed the mating system as the most important factor to shape genetic diversity and its distribution at nuclear markers (Hamrick and Godt 1996; Duminil et al. 2007). The results also confirmed previous findings that dipterocarps, like most other tropical trees, are able to avoid very low effective population sizes even if they occur in low density (Ashton 1969; Bawa 1992).
In Nanjak Makmur Sumatra, S. leprosula contained a slightly higher level of genetic diversity (H e = 0.134) than S. parvifolia (H e = 0.110), which confirmed the tendency revealed in a previous study using AFLPs (Cao et al. 2006a). Nevertheless S. leprosula showed a slightly lower level of genetic diversity (0.115) than S. parvifolia (0.122) in Sumalindo Borneo. In general, endemic tree species and species with a narrow geographic distribution harbor less genetic variation than widespread species (Hamrick et al. 1992). In the present study, however, the rare species S. blumutensis exhibited genetic diversity (H e = 0.165) higher than widespread species including scattered species S. dasyphylla and other common species. Previous studies also have shown that some rare and geographically restricted species have levels of genetic variation comparable to closely related widespread common congeners (Young and Brown 1996; Gitzendanner and Soltis 2000). These results confirmed that not all types of rarity have the same genetic implications. However, our results are based on only one rare species and need to be confirmed through studying more rare species and more populations in each species in the future.
Genetic structure
Figures 2 and 3 showed a clear separation of populations from different islands for both species S. leprosula and S. parvifolia. The pairwise F st values (0.336 for S. parvifolia, 0.404 for S. leprosula; Table 4) and the genetic distances (0.087 for S. parvifolia, 0.067 for S. leprosula; Table 5) indicated strong differentiation between populations within both species. Comparably high genetic differentiation was observed among six populations of S. parvifolia and seven populations of S. leprosula from both Borneo and Sumatra in a previous study (Cao et al. 2006a). The ecological and life history traits of the genus Shorea, in particular limited gene flow via pollen and seed dispersal, contributed to the considerable differentiation between populations. Shorea species are pollinated mainly by small insects, such as beetles and thrips, which can migrate actively only over a limited distance (Appanah and Chan 1981). Pollination predominantly within a distance of 100 m between pollen and seed parent was observed for S. acuminata (Naito et al. 2008). Pollination distances for S. leprosula were slightly longer if the density of adults was low, but mainly within 200 m (Fukue et al. 2007). Seeds of Shorea species are dispersed by wind or gravity. Although seed dispersal distances can be up to 500 m or even further, more than half of the mature seeds land within 50 m of the parent tree under forest conditions (Chan 1980; Takeuchi et al. 2004), and seed dispersal is limited even for species with comparatively light seeds and long-winged fruits (Fukue et al. 2007).
The AMOVA analysis of the present study indicated highly significant (p < 0.001) genetic differentiation among species. AFLP diversity distributed among species both in Nanjak Makmur Sumatra (57.7%) and in Sumalindo Borneo (56.3%) is higher than within-species diversity (42.3%, 43.7%, respectively; Table 3). Similarly, the overall degree of species differentiation computed using POPGENE was high in both regions (G st = 0.52 in Nanjak Makmur Sumatra, G st = 0.46 in Sumalindo Borneo). The high genetic differentiation among species is attributed to the species’ reproductive isolation from each other. However, for most species studied here, the observed levels of genetic variation are based on single populations. Hence, the significant genetic differentiation among species revealed here needs to be confirmed in further studies based on more populations for each species.
Except for three samples 2220_pc_SLB, 2217_pc_SLB, and 1221_d_NS, which are indicated with arrows in Fig. 3, the UPGMA clustering (Figs. 2 and 3) showed a very good separation of species and populations without an obvious relation to the sampling locations.
Diagnostic markers
Some markers showed very high G st values indicating their suitability to differentiate among species and between regions within species. Sequencing of these highly differentiating AFLP markers will provide specific information on homology of the same-sized AFLP fragments shared among different species and, by means of sequence comparisons to databases, on putative function of the fragments, if any. It might be possible to develop simple diagnostic markers from strongly differentiating AFLP bands. These markers may be useful as tools to unambiguously identify the species status of wood samples, and to distinguish the region of the origin of wood for widespread species (Finkeldey et al. 2007).
Equal-sized fragments may be non-homologous in different species. This is likely the case for marker 32 that distinguishes between regions for S. leprosula, but it is also present with high frequencies in S. blumutensis and S. johorensis from different regions, respectively.
Possibility of hybridization
In the UPGMA dendrogram (Fig. 3), two samples of S. platyclados from Sumalindo Borneo (2220_pc_SLB and 2217_pc_SLB) clustered together with individuals of S. parvifolia from the same region. One sample of S. dasyphylla from Nanjak Makmur Sumatra (1221_d_NS) was grouped together with individuals of S. palembanica from Sumalindo Borneo. Morphologically, leaves of these samples are distinct and easy to identify. Thus, misidentification of these samples is not very likely. S. platyclados is distributed sympatrically with S. parvifolia (Fig. 1). The habitats of S. dasyphylla and S. palembanica exist both on Sumatra and Borneo (Ashton 1982). Moreover, Shorea species flower at the same time (Appanah and Chan 1981). Although hybridization among Dipterocarpaceae species is rare, the rare event of interspecific gene exchange in Shorea is possible. In fact, interspecific hybrids among Shorea species have already been reported in former studies (Chan and Appanah 1980; Chan 1981; Ashton 1982; Harada et al. 1994; Ishiyama et al. 2003). The three samples 2220_pc_SLB, 2217_pc_SLB, and 1221_d_NS may be of hybrid origin. However, ancestral shared polymorphisms in these species cannot be excluded. A combination of species-specific markers is instrumental to detect recent hybridization events between sympatrically occurring species such as S. parvifolia, S. leprosula, and S. acuminata.
Usefulness of the AFLP technique in phylogenetic studies
AFLPs are anonymous and dominant markers. No sequence information prior to the generation of AFLP fingerprints is required, and homologous and non-homologous fragments cannot be distinguished (Mueller and Wolfenbarger 1999). Hence, size homoplasy effects possibly limit the usefulness of this marker type for phylogenetic studies. However, AFLP markers are distributed across the whole genome, and they have a high multiplex ratio, meaning a large number of different genetic loci that may be simultaneously analyzed per experiment (Pejic et al. 1998). These advantages can counteract the size homoplasy effects. Therefore, genome-wide AFLP datasets can provide high power in testing specific phylogenetic relationships in particular for closely related taxa within the same genus (Rokas et al. 2003). In closely related species, fragments of the same size are more likely to be orthologous than in distantly related species. Even though it is impossible to determine an ancestral (versus derived) character state for AFLP markers, taxonomic relationships revealed by AFLP markers are in wide accordance with the phylogenetic trees derived from sequence variation in chloroplast regions within Dipterocarpaceae (Cao et al. 2006b; Gamage et al. 2006; Indrioko et al. 2006).
Most of the phylogenetic studies based on AFLPs used only a limited number of samples per species. With our experimental design, i.e., nine species with the sample size ranging from 16 to 32, we have revealed not only pronounced within-species variation (Table 3), but also strong genetic differentiation among species indicating a strong phylogenetic signal of AFLP markers. Additionally, the UPGMA dendrogram (Fig. 3) showed a clear resolution of almost all the samples into nine species clusters based on high-frequency differences of AFLP markers among species. S. blumutensis (section Richetioides, Yellow Meranti) is isolated far from all the other species of wood variety Red Meranti. In a phylogenetic study based on the PgiC gene (Kamiya et al. 2005), section Richetioides (Yellow Meranti) was likewise basal to a clade comprising Shorea species of wood varieties Red Meranti and Balau as sister groups. In cpDNA studies (Tsumura et al. 1996; Kajita et al. 1998; Kamiya et al. 1998; Dayanandan et al. 1999), there was also a clear separation of wood varieties, but their relative position in the phylogenetic trees was not resolved. Additionally, phylogenetic studies based on cpDNA (Indrioko et al. 2006) and AFLPs (Cao et al. 2006b) differentiated genera and tribes within Dipterocarpaceae, but sectional subdivision within genus Shorea and separation of wood varieties were not reflected. AFLP fragments that strongly differentiated among species in this study will be further sequenced in order to verify the homology of the same-sized fragments.
References
Appanah S, Chan HT (1981) Thrips: the pollinators of some dipterocarps. Malay For 44:234–252
Ashton PS (1969) Speciation among tropical forest trees: some deductions in the light of recent evidence. Biol J Linn Soc 1:155–196
Ashton PS (1982) Dipterocarpaceae. Flora Males Ser I 9:237–552
Bawa KS (1992) Mating systems, genetic differentiation and speciation in tropical rain forest plants. Biotropica 24:250–255
Bouajila A, Abang MM, Haouas S, Rezgui SUS, Baum M, Yahyaoui A (2007) Genetic diversity of Rhynchosporium secalis in Tunisia as revealed by pathotype, AFLP, and microsatellite analyses. Mycopathologia 163:281–294
Cao C-P, Finkeldey R, Siregar I, Siregar U, Gailing O (2006a) Genetic diversity within and among populations of Shorea leprosula Miq. and S. parvifolia Dyer (Dipterocarpaceae) in Indonesia detected by AFLPs. Tree Genet Genome 2:225–239
Cao C-P, Gailing O, Siregar I, Indrioko S, Finkeldey R (2006b) Genetic variation at AFLPs for the Dipterocarpaceae and its relation to molecular phylogenies and taxonomic subdivisions. J Plant Res 119:553–558
Castillo-Cárdenas MF, Toro-Perea N, Cárdenas-Henao H (2005) Population genetic structure of neotropical Mangrove species on the Colombian Pacific Coast: Pelliciera rhizophorae (Pellicieraceae). Biotropica 37:266–273
Chan HT (1980) Reproductive biology of some Malaysian dipterocarps. II. Fruiting biology and seedling studies. Malay For 43:438–451
Chan HT (1981) Reproductive biology of some Malaysian dipterocarps. III. Breeding systems. Malay For 44:28–36
Chan HT, Appanah S (1980) Reproductive biology of some Malaysian dipterocarps. I. Flowering biology. Malay For 43:132–143
Dayanandan S, Ashton PS, Williams SM, Primack RB (1999) Phylogeny of the tropical tree family Dipterocarpaceae based on nucleotide sequences of the chloroplast rbcL gene. Am J Bot 86:1182–1190
De Verno LL, Mosseler A (1997) Genetic variation in red pine (Pinus resinosa) revealed by RAPD and RAPD–RFLP analysis. Can J For Res 27:1316–1320
Dice LR (1945) Measures of the amount of ecologic association between species. Ecology 26:297–302
Duminil J, Fineschi S, Hampe A, Jordano P, Salvini D, Vendramin GG, Petit RJ (2007) Can population genetic structure be predicted from life-history traits? Am Nat 169:662–672
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Excoffier L, Laval G, Schneider S (2006) ARLEQUIN version 3.01. An integrated software package for population genetics data analysis. Computational and Molecular Population Genetics Lab (CMPG), University of Berne, Switzerland. http://cmpg.unibe.ch/software/arlequin3
Finkeldey R, Rachmayanti Y, Gailing O (2007) Molecular genetic tools for the identification of the origin of wood. In: Kües U (ed) Wood production, wood technology and biotechnological impacts. Universitätsverlag Göttingen, Göttingen, Germany, pp 143–158
Fukue Y, Kado T, Lee SL, Ng KKS, Muhammad N, Tsumura Y (2007) Effects of flowering tree density on the mating system and gene flow in Shorea leprosula (Dipterocarpaceae) in Peninsular Malaysia. J Plant Res 120:413–420
Gailing O, von Wuehlisch G (2004) Nuclear markers (AFLPs) and chloroplast microsatellites differ between Fagus sylvatica and F. orientalis. Silvae Genet 53:105–110
Gamage DT, de Silva MP, Inomata N, Yamazaki T, Szmidt A (2006) Comprehensive molecular phylogeny of the sub-family Dipterocarpoideae (Dipterocarpaceae) based on chloroplast DNA sequences. Genes Genet Syst 81:1–12
Garcia AAF, Benchimol LL, Barbosa AMM, Geraldi IO, Souza CL, de Souza AP (2004) Comparison of RAPD, RFLP, AFLP and SSR markers for diversity studies in tropical maize inbred lines. Genet Mol Biol 27:579–588
Gathorne-Hardy FJ, Syaukani, Davies RG, Eggleton P, Jones DT (2002) Quaternary rainforest refugia in south-east Asia: using termites (Isoptera) as indicators. Biol J Linn Soc 75:453–466
Gitzendanner MA, Soltis PS (2000) Patterns of genetic variation in rare and widespread plant congeners. Am J Bot 87:783–792
Hamrick JL, Godt MJW (1996) Effects of life history traits on genetic diversity in plant species. Philos Trans R Soc Lond, Ser B 351:1291–1298
Hamrick JL, Godt MJW, Sherman-Broyles SL (1992) Factors influencing levels of genetic diversity in woody plant species. New For 6:95–124
Harada K, Kinoshita A, Abshukor NA, Tachida H, Yamazaki T (1994) Genetic variation estimated in three Shorea species by the RAPD analysis. Jpn J Genet 69:713–718
Hartl DL, Clark AG (1989) Principles of population genetics, 2nd edn. Sinauer, Sunderland, MA
Indrioko S, Gailing O, Finkeldey R (2006) Molecular phylogeny of Dipterocarpaceae in Indonesia based on chloroplast DNA. Plant Syst Evol 261:99–115
Isabel N, Beaulieu J, Thériault P, Bousquet J (1999) Direct evidence for biased gene diversity from dominant random amplified polymorphic DNA (RAPD) fingerprints. Mol Ecol 8:477–483
Ishiyama H, Kado T, Iwasaki M, Matsuoka M, Shukor NA, Szmidt AE, Yamazaki T (2003) Nucleotide variation in the GapC region of four species of Shorea and their putative hybrids. Tropics 13:89–99
Jones CJ, Edwards KJ, Castaglione S, Winfield MO, Sala F, van de Wiel C, Bredemeijer G, Vosman B, Matthes M, Daly A et al (1997) Reproducibility testing of RAPD, AFLP, and SSR markers in plants by a network of European laboratories. Mol Breed 3:381–390
Kajita T, Kamiya K, Nakamura K, Tachida H, Wickneswari R, Tsumura Y, Yoshimaru H, Yamazaki T (1998) Molecular phylogeny of Dipterocarpaceae in Southeast Asia based on nucleotide sequences of matK, trnL intron, and trnL–trnF intergenic spacer region in chloroplast DNA. Mol Phyl Evol 10:202–209
Kamiya K, Harada K, Kajita T, Yamazaki T, Lee HS, Ogino K, Ashton PS (1998) Molecular phylogeny of dipterocarp species using nucleotide sequences of two noncoding regions in chloroplast DNA. Tropics 7:195–207
Kamiya K, Harada K, Tachida H, Ashton PS (2005) Phylogeny of PgiC gene in Shorea and its closely related genera (Dipterocarpaceae), the dominant trees in Southeast Asian tropical rain forests. Am J Bot 92:775–788
Kremer A, Caron H, Cavers S, Colpaert N, Gheysen G, Gribel R, Lemes M, Lowe AJ, Margis R, Navarro C, Salgueiro F (2005) Monitoring genetic diversity in tropical trees with multilocus dominant markers. Heredity 95:274–280
Lara-Gomez G, Gailing O, Finkeldey R (2005) Genetic variation in isolated Mexican populations of the endemic maple Acer skutchii Rehd. Allg Forst-Jagdztg 176:97–103
Lee SL, Wickneswari R, Mahani MC, Zakri AH (2000) Mating system parameters in a tropical tree species, Shorea leprosula Miq. (Dipterocarpaceae), from Malaysian lowland Dipterocarp forest. Biotropica 32:693–702
Lewontin RC (1972) The apportionment of human diversity. In: Dobzhansky TH, Hecht MK, Steere WC (eds) Evolutinary biology. vol. 6. Appleton-Cenrury-Crofts, New York, pp 381–398
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure in plant populations. Annu Rev Ecol Syst 15:65–95
Luu HT (2005) Genetic variation and the reproductive system of Dipterocarpus cf. condorensis Pierre in Vietnam. PhD Thesis, University of Göttingen, Germany
Mantel NA (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Masum Akond ASMG, Watanabe N, Furuta Y (2008) Comparative genetic diversity of Triticum aestivum–Triticum polonicum introgression lines with long glume and Triticum petropavlovskyi by AFLP-based assessment. Genet Resour Crop Evol 55:133–141
Mueller UG, Wolfenbarger LL (1999) AFLP genotyping and fingerprinting. Trends Ecol Evol 14:389–394
Nagamitsu T, Ichikawa S, Ozawa M, Shimamura R, Kachi N, Tsumura Y, Muhammad N (2001) Microsatellite analysis of the breeding system and seed dispersal in Shorea leprosula (Dipterocarpaceae). Int J Plant Sci 162:155–159
Naito Y, Kanzaki M, Iwata H, Obayashi K, Lee SL, Muhammad N, Okuda T, Tsumura Y (2008) Density-dependent selfing and its effects on seed performance in a tropical canopy tree species, Shorea acuminata (Dipterocarpaceae). Forest Ecol Manag 256:375–383
Nason JD, Hamrick JL (1996) Reproductive and genetic consequences of forest fragmentation: two case studies of Neotropical canopy trees. J Hered 8:264–274
Nei M (1973) Analysis of gene diversity in subdivided populations. Proc Nat Acad Sci USA 70:3321–3323
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Newman MF, Burgess PF, Whitmore TC (1996a) Manuals of dipterocarps for foresters: Borneo island light hardwoods. Center for International Forestry Research, Jakarta
Newman MF, Burgess PF, Whitmore TC (1996b) Manuals of dipterocarps for foresters: Sumatra light hardwoods. Center for International Forestry Research, Jakarta
Nybom N, Bartish I (2000) Effects of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol Evol Syst 3:93–114
Obayashi K, Tsumura Y, Ihara-Ujino T, Niiyama K, Tanouchi H, Suyama Y, Washitani I, Lee CT, Lee SL, Muhammad N (2002) Genetic diversity and outcrossing rate between undisturbed and selectively logged forests of Shorea curtisii (Dipterocarpaceae) using microsatellite DNA analysis. Int J Plant Sci 163:151–158
Pejic I, Ajmone-Marsan P, Morgante M (1998) Comparative analysis of genetic similarity among maize inbred lines detected by RFLPs, RAPDs, SSRs and AFLPs. Theor Appl Genet 97:1248–1255
Rafalski JA, Vogel JM, Morgante M, Powell W, Andre C, Tingey SV (1996) Generating and using DNA markers in plants. In: Birren B, Lai E (eds) Non-mammalian genomic analysis: a practical guide. Academic, London, pp 75–134
Rogers DL (2002) In situ genetic conservation of monterey pine (Pinus radiata D. Don): information and recommendations. Genetic Resources Conservation Program, Report No. 26, University of California, Davis, CA USA, p 26
Rohlf FJ (1998) NTSYS-pc version 2.0. Numerical taxonomy and multivariate analysis system. Exeter Software, Setauket, New York
Rokas A, Williams BL, King N, Carroll SB (2003) Genome-scale approaches to resolving incongruence in molecular phylogenies. Nature 425:798–804
Sakai S, Momose K, Yumoto T, Kato M, Inoue T (1999) Beetle pollination of Shorea parvifolia (section Mutica, Dipterocarpaceae) in a general flowering period in Sarawak, Malaysia. Am J Bot 86:62–69
Symington CF (1943) Foresters’ manual of dipterocarps, Malayan forest records no. 16. Penerbit Universiti Malaya, Kuala Lumpur, Malaysia
Szmidt AE, Wang X-R, Lu M-Z (1996) Empirical assessment of allozyme and RAPD variation in Pinus sylvestris (L.) using haploid tissue analysis. Heredity 76:412–420
Takeuchi Y, Ichikawa S, Konuma A, Tomaru N, Niiyama K, Lee SL, Muhammad N, Tsumura Y (2004) Comparison of the fine-scale genetic structure of three dipterocarp species. Heredity 92:323–328
Tang SQ, Dai WJ, Li MS, Zhang Y, Geng YP, Wang L, Zhong Y (2008) Genetic diversity of relictual and endangered plant Abies ziyuanensis (Pinaceae) revealed by AFLP and SSR markers. Genetica 133:21–30
Tsumura Y, Kawahara T, Wickneswari R, Yoshimura K (1996) Molecular phylogeny of Dipterocarpaceae in Southeast Asia using RFLP of PCR-amplified chloroplast genes. Theor Appl Genet 93:22–29
Vos P, Hogers R, Bleeker M, Reijans M, Van de Lee T, Hornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Xiao M, Li Q, Guo L, Luo T, Duan W-X, He W-X, Wang L, Chen F (2006) AFLP analysis of genetic diversity of the endangered species Sinopodophyllum hexandrum in the Tibetan region of Sichuan province, China. Biochem Genet 44:47–60
Yeh FC, Yang R-C, Boyle T (1999) Popgene version 1.31. Microsoft window-based freeware for population genetic analysis. Department of Renewable Resources, University of Alberta, Edmonton, Alberta, Canada. Available free at http://www.ualberta.ca/∼fyeh/
Young AG, Brown AHD (1996) Comparative population genetic structure of the rare woodland shrub Daviesia suaveolens and its common congener D. mimosoides. Conserv Biol 10:1220–1228
Acknowledgments
The authors thank Valdir Marcos Stefenon for the kind help with data analysis and Olga Artes for technical support in the laboratory. This work was financed by the Deutsche Forschungsgemeinschaft (Fi 569/4-1/2) and the Bundesministerium für wirtschaftliche Zusammenarbeit (BMZ).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Y. Tsumura
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Cao, CP., Gailing, O., Siregar, I.Z. et al. Genetic variation in nine Shorea species (Dipterocarpaceae) in Indonesia revealed by AFLPs. Tree Genetics & Genomes 5, 407–420 (2009). https://doi.org/10.1007/s11295-008-0195-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11295-008-0195-4