Abstract
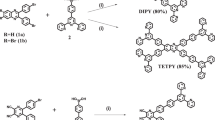
We have successfully prepared two ruthenium-based covalent bonding photosensitizer–catalyst dyads through a simple procedure. 1H NMR spectra of both dyads show that only a single stereoisomer was formed for each dyad. The spectroscopic and electrochemical properties and photocatalytic water oxidation activities of both dyads were investigated in detail. The results indicate that there is negligible electron communication between the photosensitizer and catalyst centers, and each component maintains the desired photophysical and electrochemical properties, which would diminish excited-state electron recombination by facilitating the intramolecular electron transfer. In the presence of excess sacrificial electron acceptor, the dyad with iodide ligand shows a 5.5-fold increase in catalytic performance as compared to its chloro analogue, indicating that the iodide ligand plays an important role during the catalytic cycle. Moreover, compared with the multi-component system, the dyad with iodide ligand exhibits a fourfold increase in catalytic turnover number.
Graphical abstract







Similar content being viewed by others
References
Yagi M, Kaneko M (2001) Chem Rev 101:21–36
Lewis NS, Nocera DG (2006) Proc Natl Acad Sci USA 103:15729–15737
Barber J (2009) Chem Soc Rev 3:185–196
Li T-T, Chen Y, Li F, Zhao W, Wang C, Lv X, Xu Q, Fu W (2014) Chem Eur J 20:8054–8061
Li T-T, Qian J, Zheng Y-Q (2016) RSC Adv 6:77358–77365
Meyer K, Ranocchiari M, van Bokhoven JA (2015) Energy Environ Sci 8:1923–1937
Blakemore JD, Crabtree RH, Brudvig GW (2015) Chem Rev 115:12974–13005
Karkas MD, Verho O, Johnston EV, Åkermark B (2014) Chem Rev 114:11863–12001
Li T-T, Zheng Y-Q (2016) Dalton Trans 45:12685–12690
Limburg B, Bouwman E, Bonnet S (2012) Coord Chem Rev 256:1451–1467
Singh A, Spiccia L (2013) Coord Chem Rev 257:2607–2622
Li F, Jiang Y, Zhang B, Huang F, Gao Y, Sun L (2012) Angew Chem Int Ed 51:2417–2420
Sun L, Hammarström L, Åkermarka B, Styring S (2001) Chem Soc Rev 30:36–49
Ashford DL, Stewart DJ, Glasson CR, Binstead RA, Harrison DP, Norris MR, Concepcion JJ, Fang Z, Templeton JL, Meyer TJ (2012) Inorg Chem 51:6428–6430
Norris MR, Concepcion JJ, Harrison DP, Binstead RA, Ashford DL, Fang Z, Templeton JL, Meyer TJ (2013) J Am Chem Soc 135:2080–2083
Kaveevivitchai N, Chitta R, Zong R, Ojaimi ME, Thummel RP (2012) J Am Chem Soc 134:10721–10724
Kohler L, Kaveevivitchai N, Zong R, Thummel RP (2014) Inorg Chem 53:912–921
Nair NV, Zhou R, Thummel RP (2017) Inorg Chim Acta 454:27–39
Li T-T, Li F-M, Zhao W-L, Tian Y-H, Chen Y, Cai R, Fu W-F (2015) Inorg Chem 54:183–191
Broomhead JA, Young CG (1982) Inorg Synth 21:127–128
Takeuchi KJ, Thompson MS, Pipes DW, Meyer TJ (1984) Inorg Chem 23:1845–1851
Kaveevivitchai N, Zong R, Tseng H-W, Chitta R, Thummel RP (2012) Inorg Chem 51:2930–2939
McClanahan SF, Dallinger RF, Holler FJ, Kincaid JR (1985) J Am Chem Soc 107:4853–4860
Herrero C, Quaranta A, Fallahpour RA, Leibl W, Aukauloo A (2013) J Phys Chem C 117:9605–9612
Swavey S, Fang Z, Brewer KJ (2002) Inorg Chem 41:2598–2607
Hamelin O, Guillo P, Loiseau F, Boissonnet M-F, Menage S (2011) Inorg Chem 50:7952–7954
Canterbury TR, Arachchige SM, Moore RB, Brewer KJ (2015) Angew Chem Int Ed 54:12819–12822
Herrero C, Quaranta A, Leibl W, Rutherford AW, Aukauloo A (2011) Energy Environ Sci 4:2353–2365
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21603110) and K. C. Wong Magna Fund in Ningbo University. Y. Z. thanks the support from K. C. Wong Education Foundation, Hong Kong.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Q., Zhou, Q., Li, TT. et al. Covalent bonding photosensitizer–catalyst dyads of ruthenium-based complexes designed for enhanced visible-light-driven water oxidation performance. Transit Met Chem 44, 349–354 (2019). https://doi.org/10.1007/s11243-018-00301-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-018-00301-3